Histopathological patterns in atypical teratoid/rhabdoid tumors are related to molecular subgroup
Abstract
Atypical teratoid/rhabdoid tumor (AT/RT) is a highly malignant tumor that may not only contain rhabdoid tumor cells but also poorly differentiated small-round-blue cells as well as areas with mesenchymal or epithelial differentiation. Little is known on factors associated with histopathological diversity. Recent studies demonstrated three molecular subgroups of AT/RT, namely ATRT-TYR, ATRT-SHH, and ATRT-MYC. We thus aimed to investigate if morphological patterns might be related to molecular subgroup status. Hematoxylin-eosin stained sections of 114 AT/RT with known molecular subgroup status were digitalized and independently categorized by nine blinded observers into four morphological categories, that is, “rhabdoid,” “small-round-blue,” “epithelial,” and “mesenchymal.” The series comprised 48 ATRT-SHH, 40 ATRT-TYR, and 26 ATRT-MYC tumors. Inter-observer agreement was moderate but significant (Fleiss’ kappa = 0.47; 95% C.I. 0.41-0.53; p < 0.001) and there was a highly significant overall association between morphological categories and molecular subgroups for each of the nine observers (p < 0.0001). Specifically, the category “epithelial” was found to be over-represented in ATRT-TYR (p < 0.000001) and the category “small-round-blue” to be over-represented in ATRT-SHH (p < 0.01). The majority of ATRT-MYC was categorized as “mesenchymal” or “rhabdoid,” but this association was less compelling. The specificity of the category “epithelial” for ATRT-TYR was highest and accounted for 97% (range: 88-99%) whereas sensitivity was low [49% (range: 35%–63%)]. In line with these findings, cytokeratin-positivity was highly overrepresented in ATRT-TYR. In conclusion, morphological features of AT/RT might reflect molecular alterations and may also provide a first hint on molecular subgroup status, which will need to be confirmed by DNA methylation profiling.
1 INTRODUCTION
Atypical teratoid/rhabdoid tumor (AT/RT) is a highly malignant central nervous system tumor mainly occurring in infancy and childhood (1). The designation “teratoid/rhabdoid” coined by Lucy B. Rorke-Adams (2, 3) reflects the notion that AT/RT may not only contain rhabdoid cells similar to malignant rhabdoid tumors of the kidney but also poorly differentiated small round blue cells as well as areas of mesenchymal or epithelial differentiation. The variety of morphological patterns represented a diagnostic problem and some also argued that such heterogeneity could not be compatible with a distinct entity (4). The immunohistochemical staining profile of AT/RT is also diverse with frequent positivity for vimentin and epithelial membrane antigen, but a proportion of cases also expressing cytokeratin, actin, GFAP, and/or neuronal markers (3). However, it soon became obvious that bi-allelic mutations of SWI/SNF chromatin remodeling complex member SMARCB1 (also known as hSNF5/INI1) are a characteristic genetic lesion (5, 6). The resulting loss of nuclear SMARCB1 protein expression (7) has been successfully employed to establish the diagnosis of AT/RT also in embryonal tumors lacking rhabdoid features (8, 9) and is nowadays routinely used to distinguish AT/RT from other malignant pediatric brain tumors. The diversity of morphological patterns in AT/RT, however, remained enigmatic, and little is known on associated clinical, genetic, or epigenetic factors.
Recent studies demonstrated three molecular subgroups of AT/RT, namely ATRT-TYR, ATRT-SHH, and ATRT-MYC (10, 11). These molecular subgroups are characterized by distinct DNA methylation signatures, gene expression profiles, and clinical features (12). Because molecular subgrouping has a prognostic role (13) and may predict treatment-specific response and survival in ATRT patients (14), it can be expected to become a standard of care in the near future. Here we show that the diversity of morphological patterns in AT/RT is related to molecular subgroup status.
2 MATERIALS AND METHODS
2.1 Tumor samples
Material from representative paraffin blocks from 114 SMARCB1-deficient AT/RT that had been sent for central review in the context of the European Rhabdoid Registry EU-RHAB was retrieved from the archives of the Institute of Neuropathology Münster (Table 1). EU-RHAB and the tumor bank of the Institute of Neuropathology Münster have received continuous local ethics committee approval (Ethics committee of the University Hospital Münster), and patients or the guardians gave informed consent for the scientific use of archival materials. The diagnosis of AT/RT was confirmed using current WHO criteria and routinely included the demonstration of loss of tumoral SMARCB1/INI1 protein expression using immunohistochemistry. DNA methylation profiles have been published previously (13) and were generated using the Methylation EPIC BeadChip array (Illumina, San Diego, CA) and DNA methylation-based classification (15) using the Heidelberg Brain Tumor Classifier (version v11b4) and confirmatory t-distributed stochastic neighbor embedding (TSNE) analyses.
| Age [median (interquartile range)] | 18 (10–26) months |
| Sex (male/female) | 63/51 |
| Tumor location | |
| Supratentorial | 58 (51%) |
| Infratentorial | 53 (46%) |
| Spinal | 1 (<1%) |
| Several locations | 1 (<1%) |
| Not available | 1 (<1%) |
| Molecular subgroup | |
| ATRT-TYR | 40 (35%) |
| ATRT-SHH | 48 (42%) |
| ATRT-MYC | 26 (23%) |
Note
- Clinical and molecular features of 114 AT/RT cases.
2.2 Image acquisition and rating
Images of hematoxylin-eosin stained slides were digitally acquired on a Leica SCN400 Slide Scanner up to a 400x magnification and uploaded to an OMERO online platform [version 5.6.2 www.openmicroscopy.org; (16)]. For all slides, tissue area was quantified using the QuPath Software (version 0.2.3) by applying the “simple tissue detection” algorithm at constant parameters.
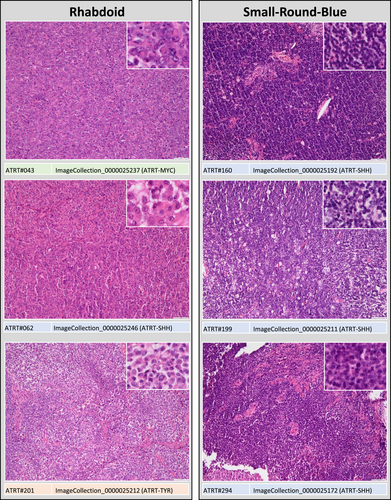
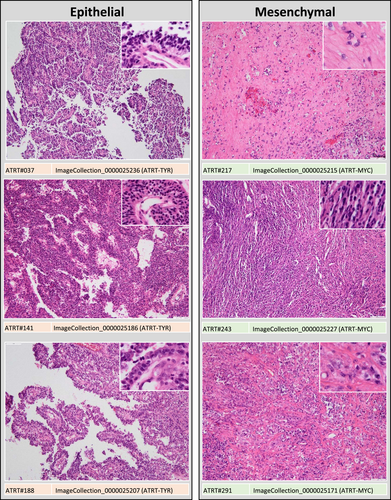
For evaluation of morphological patterns, slides were independently reviewed by nine observers (four residents and five board-certified neuropathologists). Observers received instructions and a training set of digitalized slides and were then asked to categorize each AT/RT sample in one of four morphological categories: (1) “small-round-blue” comprising AT/RT mainly (>50%) composed of small round blue cells, (2) “mesenchymal” comprising AT/RT with a prevalence (>50%) of spindled cells, desmoplasia and/or myxoid changes, (3) “epithelial,” comprising AT/RT with epithelial features (i.e., formation of surfaces, intratumoral lumina or loosely dehiscent papillary structures) and (4) “rhabdoid” for those AT/RT, in which rhabdoid cells prevailed (>50%; see Figures 1 and 2). Observers were blinded to molecular subgroup status.
2.3 Immunohistochemistry
Immunohistochemical staining for cytokeratin (MNF-116, Dako M0821, 1:800, proteinase K pretreatment) was performed using the streptavidin-biotin method on an automated staining system (Omnis, DAKO). In a proportion of cases staining results for epithelial membrane antigen, vimentin, actin, GFAP, synaptophysin, and tyrosinase were also available for evaluation.
2.4 Statistics
Associations between morphological or immunohistochemical categories and molecular subgroup status were examined using chi-square test and the distribution of age and percentages of cytokeratin-positive tumor cells across morphological categories using nonparametric ANOVA. Inter-observer agreement was determined with Fleiss´ kappa. Specificities and sensitivities were determined for each individual observer and are given as means (range) for the nine observers. Statistical analyses were performed using SPSS (Version 27) and the irr and boot packages in R (Version 3.6.3). A p value <0.05 was considered significant.
3 RESULTS
The median tissue area represented on the slides accounted for 1.58 cm2 (interquartile range: 0.75−2.69 cm2), suggesting that the size of the tissue samples was sufficient for morphological evaluation. The nine observers categorized 34% [26%–46%; mean(range)] of the AT/RT samples as “small-round-blue,” 19% (13-29%) as “epithelial,” 25% (12-44%) as “mesenchymal” and 21% (12%–32%) as “rhabdoid” (Figures 1 and 2). Inter-observer agreement was moderate but highly significant [Fleiss’ kappa = 0.47 (95% confidence interval: 0.41–0.53); p < 0.001] and no systematic differences between four novice and five experienced reviewers were observed (Figure 3A,C,E). There was no difference in tumor location between cases rated by the majority of observers as “rhabdoid,” “small-round-blue,” or “mesenchymal,” but a predilection of “epithelial” cases for infratentorial location was noted (chi-square 7.425, df1, p = 0.006). Median age was 20.5 months for “rhabdoid,” 18 months for “small-round-blue,” 21 months for “mesenchymal” and only 11.5 months for “epithelial” cases, but this difference did not reach significance.
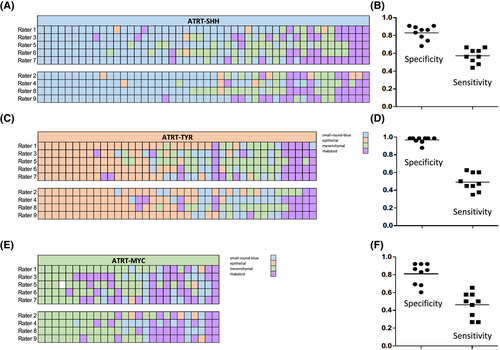
The series comprised 48 ATRT-SHH, 40 ATRT-TYR, and 26 ATRT-MYC (for details see Table S1). There was a highly significant overall association between morphological categories and molecular subgroup status for all nine observers (p < 0.0001; Table S2). Specifically, the category “small-round-blue” was over-represented in ATRT-SHH (Figure 3A, p < 0.01) and the category “epithelial” was over-represented in ATRT-TYR (Figure 3C, p < 0.000001). The majority of ATRT-MYC were categorized as “mesenchymal” or “rhabdoid,” but this association was less convincing, since only 5/9 observers assigned the category “mesenchymal” significantly more frequently to ATRT-MYC (Figure 3E).
The diagnostic specificity of the morphological category “epithelial” for ATRT-TYR accounted for 97% (mean, range: 88%–99%, Figure 3B), whereas specificities of the categories “small-round-blue” for ATRT-SHH and “mesenchymal” for ATRT-MYC accounted for 83% and 81%, respectively (Figure 3D,F). The sensitivities of “epithelial” for ATRT-TYR, small-round-blue” for ATRT-SHH and “mesenchymal” for ATRT-MYC were relatively low and accounted for 49%, 57%, and 46%, respectively.
In order to further validate the association of epithelial features and ATRT-TYR, immunohistochemistry for cytokeratin (MNF116) was performed in 107 cases, for which material was available. In line with previous observations (3), a substantial proportion of AT/RT [61/107 (57%)] showed positivity for cytokeratin, often highlighting surfaces but also rhabdoid tumor cells (Figure S1A–C). The proportion of cytokeratin-positive cases was significantly higher in ATRT-TYR as compared to ATRT-SHH and ATRT-MYC (Chi-Square = 35.801, df = 2, p = 0.00000008). The median percentage of cytokeratin-positive tumor cells was also significantly higher in ATRT-TYR (30.5%) as compared to ATRT-SHH (0%) and ATRT-MYC (3%; Figure S1D). Additional immunohistochemical staining results were available for a proportion of cases (for details see Table S1). All tumors examined stained positive for epithelial membrane antigen (54/54) and vimentin (10/10) and there was no significant difference among molecular subgroups in the proportion of cases positive for actin [ATRT-TYR: 63%, ATRT-SHH: 63%, ATRT-MYC: 75% (Chi-Square: 0.22 df:2 p = 0.89)], GFAP [ATRT-TYR: 62%, ATRT-SHH: 41%, ATRT-MYC: 33% (Chi-Square: 1.783 df:2 p = 0.41)] and synaptophysin [ATRT-TYR: 70%, ATRT-SHH: 50%, ATRT-MYC: 33% (Chi-Square: 1.596 df:2 p = 0.45)]. In line with our previous observations (12, 17), tyrosinase expression was highly over-represented in the ATRT-TYR molecular subgroup [ATRT-TYR: 83%, ATRT-SHH: 4%, ATRT-MYC: 0% (Chi-Square: 74.022 df:2 p = 1.0E-10)].
4 DISCUSSION
The finding that morphological patterns in AT/RT are related to molecular subgroup status suggests that histopathological features might reflect underlying molecular alterations.
The frequency of AT/RT categorized as “small-round-blue,” “mesenchymal,” “epithelial,” and “rhabdoid” resembles the first large series of 52 AT/RT reported by Rorke-Adams (3). In that series, cases showing small round-blue-cell areas were also most frequent (67%), a mesenchymal component was present in 31% of tumors, epithelial features were encountered in 25% of tumors, and 13% solely consisted of rhabdoid tumor cells (3). Our findings are also well in line with other previous histopathological studies, in which small-round-blue-cell areas were most frequently encountered (18, 19). The authors of one recent series also noted differences of histopathological patterns according to clinical features with tumors showing small-round-blue-cell areas being more frequent in younger children (20). Even though molecular subgroup status was not examined in that study, this finding might well point towards differences of histopathological patterns among molecular subgroups, since patients harboring ATRT-MYC are significantly older (12) and in the present series also rarely were categorized as “small-round-blue.” The subunits of the BAF and PBAF SWI/SNF complexes are central regulators of lineage specification during development. Alterations in these subunits have recently been shown to correlate with the neuronal or epithelial and mesenchymal differentiation in AT/RT (21).
Cases categorized as small-round-blue-cell were overrepresented in ATRT-SHH. The molecular subgroup ATRT-SHH is characterized by a neural expression signature with pathway activation of sonic hedgehog (SHH) and Notch (12). Other small-round-blue-cell tumor entities also display neural expression profiles, suggesting that the predominance of a small-round-blue-cell phenotype in ATRT-SHH might well represent a reflection of its neural expression signature.
Samples categorized as epithelial were overrepresented in ATRT-TYR. ATRT-TYR is characterized by overexpression of tyrosinase (12, 17), which is an important protagonist in neural tube development (22). Our finding that positivity for epithelial marker cytokeratin was also overrepresented in ATRT-TYR, further suggests a link between ATRT-TYR and epithelial differentiation. We have recently shown that cribriform neuroepithelial tumor (CRINET), a rare non-rhabdoid SMARCB1-deficient brain tumor with neuroepithelial histopathology and relatively favorable prognosis (23) shows epigenetic similarities with ATRT-TYR (24). The finding that epithelial features were also over-represented in ATRT-TYR further suggests similarities of ATRT-TYR and CRINET. As further data become available for these rare tumors it will be interesting to learn whether they represent a unique developmental endpoint of ATRT-TYR.
Finally, ATRT-MYC were often categorized as mesenchymal or rhabdoid, possibly reflecting the epigenetic similarities of this molecular subgroup with extracranial malignant rhabdoid tumors (25), which in addition to a rhabdoid phenotype may also display areas rich in connective tissue and spindled tumor cells (26).
These findings could also be of diagnostic value. Especially the specificity of epithelial features for ATRT-TYR was high. If present, epithelial features might thus give a first hint on the possibility of ATRT-TYR, which will need to be further confirmed by subsequent DNA methylation profiling (12). The same holds true for the diagnostic value of small-round-blue-cell areas for ATRT-SHH and (to a lesser extent) mesenchymal areas for ATRT-MYC.
One strength of the study is the relatively large number of independent observers. These included experts and trainees from various institutions, suggesting that the results are realistic and could also be applicable in a routine diagnostic setting. We aimed at keeping the rating system simple and chose only a few morphological categories in order to increase the reproducibility of our findings. Even though tissue sections available for morphological examination were relatively large, sampling bias due to tumor heterogeneity cannot be entirely excluded. The fact that the majority of AT/RT contains divergent and transitional histopathological patterns certainly is also contributing to only moderate interobserver agreement. Unsupervised machine learning (27) could be a promising approach to this problem and might aid to identify morphological features associated with molecular subgroups more reproducibly. However, the number of samples for a training cohort would ideally exceed 100 cases per molecular subgroup, a number difficult to achieve especially for ATRT-MYC, which represents only 23% of these rare tumors.
In conclusion, the diversity of morphological patterns in AT/RT is related to molecular subgroup status. Our findings suggest that histopathological features of AT/RT might reflect molecular alterations and may also provide a first hint on molecular subgroup status, which will need to be further confirmed by subsequent DNA methylation profiling.
ACKNOWLEDGMENTS
We thank Dr. Thomas Zobel and the Imaging Network of the Cells in Motion Interfaculty Centre, Westfälische Wilhelms-Universität Münster) for kind support and hosting the OMERO image server, Klavs Grantins (Gerhard-Domagk-Institute of Pathology, University Hospital Münster) for digital slide acquisition and Dr. Raphael Koch (Institute of Biostatistics and Clinical Research, University Hospital Münster) for statistical assistance. This study was supported by DFG (HA 3060/8-1) and IZKF Münster (Ha3/017/20). U.S. is supported by DFG, Wilhelm Sander Stiftung and Fördergemeinschaft Kinderkrebszentrum Hamburg.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
AUTHOR CONTRIBUTIONS
Martin Hasselblatt designed the study together with Francesca Zin. Francesca Zin, Jennifer A. Cotter, Christine Haberler, Matthias Dottermusch, Julia Neumann, Ulrich Schüller, Leonille Schweizer, Christian Thomas, Karolina Nemes, Pascal D. Johann, Marcel Kool, Michael C. Frühwald, Werner Paulus, Alexander Judkins, and Martin Hasselblatt were involved in the acquisition, analysis, or interpretation of the data. Francesca Zin drafted the manuscript and all co-authors revised it critically for intellectual content.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are provided in the Supporting Information or are available from the corresponding author upon reasonable request.




