Sickle cell anaemia therapy in 2025
Summary
New pharmacological therapies for sickle cell anaemia have not been as efficacious as hoped, while widespread application of curative stem cell and gene therapies is not likely to occur soon. This situation raises the question about whether more attention now be devoted to the use of hydroxyurea in this disease.
After years of relative neglect, potential therapies for sickle cell disease are being pursued by many investigators.1 Indeed, these efforts are now being regularly reported in the news media. Despite these enthusiasms, several recent events have called into question some of the assumptions underlying the belief that very effective therapies, or even cures, are near for many patients suffering from the sickle cell disease syndromes.
First, the efficacy of two of the only four drugs approved by the U.S. Food and Drug Agency (FDA) for pharmacological treatment of this disease has recently been questioned if not negated. Crizanlizumab, a Novartis inhibitor of P-selectin-mediated adhesion of sickle cells, lost its accreditation by the European Medicines Agency in May 2023.2 In September 2024, Pfizer, Inc. withdrew Voxelotor from the market based on evidence from post-marketing studies of harm due to administration of this agent,3 despite haematocrit increases. Second, progress in several of the FDA-approved approaches to gene therapy in this disease (and others) has recently been questioned as they, along with stem cell therapies, may also increase the risk of secondary neoplasms due to necessary marrow ablation procedures.4 Moreover, their extremely high costs5 as well as limited hospital facilities for such therapies—even in high-resource settings—are also likely to severely limit their applicability for some time.
Furthermore, although mortality of children in the United States with sickle cell anaemia has been markedly reduced in recent years, age-adjusted mortality of adults with this condition has been reported to have increased since 2000.6, 7 This may be partially due to the accumulated chronic organ damage in surviving children despite therapies which leads to increased mortality as adults. However, it may also reflect the widely acknowledged uncertainty of continued access to specialized care in the United States for sickle cell patients after adolescence8; a situation which clearly needs improvement.
What lessons can be learned from the new developments regarding these several therapies for sickle cell disease? Are the problems revealed by these drug failures linked to therapeutic strategies that relate to other disease-related phenomena as alternatives to focusing on the ‘classical’ understanding of the pathophysiology of sickle cell disease associated with intracellular deoxygenated sickle haemoglobin polymerization? More emphasis could also be put on developing therapies that can be used widely and inexpensively and soon—especially oral agents—and less on the more abstract, though scientifically exciting, goals that may well take many more decades for widespread application. The excellent research and clinical enterprises available may thus be able to help many more patients in the immediate future than is likely with the paradigms that have appeared to be prevalent in sickle cell research in the last few decades.
From the 1960s on, biophysical studies indicated that it was the impaired deformability of sickle haemoglobin polymer-containing erythrocytes which caused impaired cell transits in the microcirculation (including in its arteriolar section) and resulting reduced oxygen delivery to tissues and suggested that relieving this sequence should be the object for therapeutic approaches. These processes have been studied kinetically and under equilibrium conditions and showed that such polymer (or aggregation) formation occurs even at very high oxygen saturation values.9 Furthermore, sickle haemoglobin polymerization tendency, determined by intracellular haemoglobin concentrations and other red cell parameters, correlates well with overall severity of the various sickle syndromes.10
Factors that determined arteriolar resistance to blood flow by rigid sickle cells are also thought to be directly relevant to disease manifestations. Haemolysis of sickle cells probably also contributes in several ways to primary aspects of disease pathology but simple reduction of this—due to increased oxygen affinity and the resulting increasing haematocrit—may have complex effects on the disease in many patients, as the recent experience with Voxelotor suggests. Figure 1 shows diagrammatically a current understanding of sickle cell pathophysiology. As noted, research on interfering with these abnormal processes constituted the bulk of therapeutic approaches until the last few decades; they may be considered to constitute the essential pathophysiological factors that are now called, in genetic epidemiology, endophenotypes.
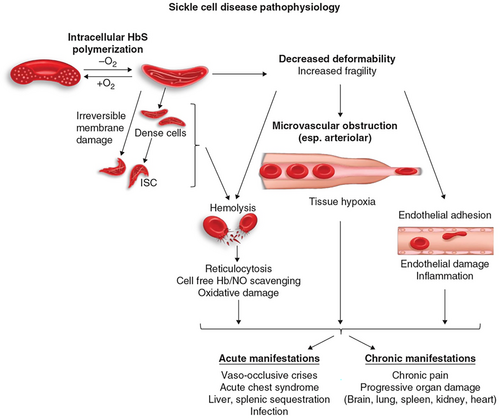
However, in the last few decades, many other phenomena that could be detected in studying sickle cell blood, such as increased adhesive properties of sickle red cells, increased leucocyte numbers or granulocyte nets or evidence of inflammatory processes, were reported11 and have also been studied as a basis for therapeutic approaches. Although interfering with these processes may be of some benefit to sickle cell patients, it is unclear that these effects would suffice to reduce the morbidity and to increase the longevity of most sickle cell anaemia patients.
Only one agent based on the classical paradigm noted above has been FDA approved (in 1998) for oral pharmacological use in sickle cell anaemia: hydroxyurea (hyroxycarbamide). This drug increases fetal haemoglobin levels, a result which had been shown in extensive cellular and biophysical studies to inhibit sickle haemoglobin polymerization. Multiple clinical studies also showed strong correlations of fetal haemoglobin levels with milder sickle disease. Phase one studies of hydroxyurea suggested that it could safely raise fetal haemoglobin levels significantly in most (but not all) patients.12, 13 A subsequent large, controlled multicentre study of hydroxyurea therapy confirmed major overall benefits for many clinical manifestations (e.g. hospitalizations, blood transfusions, acute chest syndrome) in sickle cell anaemia patients.14
Despite its known efficacy, low cost and relative ease of administration, the use of hydroxyurea has been very limited, especially for most adults.15 Concerns about hydroxyurea dosing schedules and both variable and slow fetal haemoglobin responses, as well as the need to monitor blood assays (including fetal haemoglobin levels) may have contributed to this underutilization, as did initial concerns about possible immediate and delayed side effects—including bone marrow and spermatogenesis suppression—and nausea. Recent studies have not found evidence for teratogenesis related to hydroxyurea therapy and have even suggested that this drug may, with informed consent, be considered for continuation during later parts of pregnancy.16 However, the continued tepid response of the medical community across the world, and even of the adult patient population themselves, to the availability of this agent is not fully understood.
More focus should now be placed on improving the utility of hydroxyurea in sickle cell disease. Multiple studies have suggested that hydroxyurea reduces overall mortality and lengthens life span,17 but more rigorous controlled studies of these conclusions are still needed. The Baby HUG study showed its efficacy in treating children18 and it is now more widely used in paediatric sickle cell clinics, even for very young children. Recent studies in Sub-Saharan Africa should set a benchmark for further studies in areas with scant resources but high prevelance of the sickle cell syndromes. This work has confirmed the feasibility of widespread use of hydroxyurea and its potential benefits for these communities,19 including in possible resistance to malaria.20 Indeed, some of the African clinical trials networks might also be developed, on a semi-permanent basis, in areas of higher resources, as the U.S. National Institutes of Health pioneered decades ago.
Patients might also benefit from developing other oral agents to increase fetal haemoglobin, as noted in a recent commentary in Science,21 accompanying the report of a potential new relevant epigenetic pathway. We now understand that such agents are only likely to be efficacious if they can achieve fairly pan-cellular levels of fetal haemoglobin, above about 25% of red cell haemoglobin species, at acceptably safe dosing. Studies of other ways to directly improve the flow of sickle haemoglobin polymer-containing cells in the microcirculation would also seem to deserve high priority. Continued primary attention to the many other abnormal phenomena that can be detected in the blood of sickle cell patients may be much less likely to result in the strong therapeutic results that are more related to interfering with the primary pathophysiological mechanisms.
Indeed, use of pain, that is, vaso-occlusive crises (VOC's), as the prime or even only parameter for judging success of an agent for approval as a medication, in contrast to measures of organ damage or mortality itself has not been validated. First, persistence of pain has been noted even in individuals apparently genetically ‘cured’ of their disease. Second, it is not clear that various clinical attributes always track together or that lowering the frequency or duration of VOC's will correct many of the other serious manifestations of this disease. Further studies on the relationships among these disease manifestations—stroke, acute chest syndrome, pulmonary hypertension, cardiomyopathy, renal failure, etc.—with VOCs are also very much needed in developing new therapeutic regimens. The results of such studies may suggest that multiple therapies be used in combination for any one patient or at different times in their lifespan.
Lastly, and perhaps also most relevant to current academic studies of many diseases, it is clear that improvements in stem cell therapies and gene therapies are occurring rapidly, especially with new tools such as CRISPR, and will ultimately change the very nature of many medical treatments, especially among genetic diseases. However, these are currently all very difficult and expensive approaches, whose long-term safety, clinical durability and general applicability will take, even after all the work of the last several decades, many more years to clarify. The research community should balance emphasis on these long-term ‘curative’ approaches with focus on treatments that are likely to be applicable in the very near future for a much larger fraction of patients even in resource-rich countries, if not the world, as current estimates for sickle cell births are close to 500 000 per year.
AUTHOR CONTRIBUTIONS
ANS conceptualized, researched, wrote, discussed the manuscript with knowledgeable individuals, and revised and approved the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, Bethesda, Maryland in the United States. The author thanks Drs. Mark T. Gladwin, Constance T. Noguchi, Geraldine P. Schechter and Babette B. Weksler for discussions and comments on the manuscript.
FUNDING INFORMATION
Intramural Research Program of the National Institute of Diabetes, Digestive, and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA, Project ZIA DK025016-50.
CONFLICT OF INTEREST STATEMENT
The author declares no competing interests.
PERMISSION TO REPRODUCE MATERIAL
Not relevant as figure was produced at NIH and is not under copyright.




