Acute chest syndrome in adult patients with sickle cell disease: The relationship with the time to onset after hospital admission
Summary
Data on acute chest syndrome (ACS) in adult sickle cell disease patients are scarce. In this study, we describe 105 consecutive ACS episodes in 81 adult patients during a 32-month period and compare the characteristics as a function of the time to onset after hospital admission for a vaso-occlusive crisis (VOC), that is early-onset episodes (time to onset ≤24 h, 42%) versus secondary episodes (>24 h, 58%; median [interquartile range] time to onset: 2 [2–3] days). The median age was 27 [22–34] years, 89% of the patients had an S/S or S/β0-thalassaemia genotype; 81% of the patients had a history of ACS (median: 3 [2–5] per patient), only 61% were taking a disease-modifying treatment at the time of the ACS. Fever and chest pain were noted in respectively 54% and 73% of the episodes. Crackles (64%) and bronchial breathing (32%) were the main abnormal auscultatory findings. A positive microbiological test was found for 20% of episodes. Fifty percent of the episodes required a blood transfusion; ICU transfer and mortality rates were respectively 29% and 1%. Secondary and early-onset forms of ACS did not differ significantly. Disease-modifying treatments should be revaluated after each ACS episode because the recurrence rate is high.
-
- Abbreviations: ACS
-
- acute chest syndrome
-
- CSSCD
-
- Cooperative Study for Sickle Cell Disease
-
- CRP
-
- C-reactive protein
-
- CT
-
- computed tomography
-
- HU
-
- hydroxyurea
-
- ICU
-
- intensive care unit
-
- IQR
-
- interquartile range
-
- MCV
-
- mean corpuscular volume
-
- NACSSG
-
- National Acute Chest Syndrome Study Group
-
- PE
-
- pulmonary embolism
-
- RBC
-
- red blood cell
-
- RR
-
- respiratory rate
-
- SCD
-
- sickle cell disease
-
- VOC
-
- vaso-occlusive crisis.
INTRODUCTION
Acute chest syndrome (ACS) is one of the most severe complications observed in adult patients with sickle cell disease (SCD) and is associated with high morbidity and mortality rates.1 The syndrome is conventionally defined in France as a combination of one or more respiratory symptoms (chest pain, dyspnoea, cough, expectoration, fever) or abnormal auscultation findings and a new pulmonary infiltrate on imaging.2 Although ACS commonly occurs after a bone vaso-occlusive crisis (VOC), it may also be inaugural.3 Relative to a VOC alone, ACS is associated with a significantly longer length of hospital stay, a greater likelihood of admission to an intensive care unit (ICU), and a greater frequency of red blood cell (RBC) transfusion.3-6 Hence, ACS is a major cause of death in people with SCD1, 7-11; the mortality rate ranges from 1.6% to 4.6% in hospitalized patients not admitted to the ICU12, 13 and can be as high as 13% in patients admitted to the ICU.14, 15
Despite its severity, precise epidemiological data on ACS are scarce. The prevalence of ACS after a VOC (i.e. secondary ACS) ranges from 12.5% to 42%, and the median time to onset is usually between 2–4 days.3, 4, 16-20 However, data on early-onset ACS (i.e. onset less than 24 hours (h) after the VOC) are particularly scarce, and it is not known whether early-onset ACS and secondary ACS have different characteristics. Indeed, the pathophysiology of ACS is complex, and one can hypothesize that early-onset ACS often has an infectious trigger.
Thus, the objectives of the present study were to (i) describe the clinical, laboratory and imaging characteristics of consecutive episodes of ACS, and (ii) compare early-onset ACS with secondary ACS after hospital admission for a VOC.
MATERIALS AND METHODS
Participants
We conducted a single-centre study at the French national referral centre for adults with SCD (Georges Pompidou European Hospital, Paris, France). All consecutive inpatient stays by adults (aged 18 and over) with diagnoses of SCD and ACS between April 24th, 2017, and December 31st, 2019, were included in the study. During this period, all ACS episodes were registered prospectively by the director of the referral centre after careful examination of the patients' medical charts. The director produced a weekly summary of all episodes of ACS that had occurred in the hospital. Data were collected retrospectively. After a review of the medical charts, hospital stays corresponding to patients without clinical or radiological features of ACS, or infectious pneumonia (microbiologically proven) without vaso-occlusive manifestations, were excluded from the analysis.
Definition of ACS
ACS was defined according to the French national guidelines as a combination of at least one respiratory symptom or physical sign (fever, chest pain, cough, expectoration, dyspnoea, crackles, bronchial breathing, decreased vesicular breath sound) and a new pulmonary infiltrate on imaging (chest X-ray or computed tomography (CT) scan), other than atelectasis,2 in the absence of alternative cause such as volume overload. Radiological diagnosis of ACS and description of their characteristics was obtained either using the radiologists' report for CT scan, or by interpretation of chest X-ray by one of us (GC) who collected the data (no radiologist report available for chest X-ray in our hospital). Severe ACS was defined as the presence of at least one objective clinical, laboratory or radiological severity criterion (according to the French national guidelines)2 (Table S5), transfer to the ICU, or death. Episodes of ACS were classified as early-onset (respiratory signs starting 24 h or less after hospital admission) or secondary (respiratory signs starting >24 h after admission). The time to onset (diagnosis) of ACS was defined as the time of occurrence of the first compatible clinical sign or the time of occurrence of the first radiological infiltrate, if the latter was followed by clinical compatible signs within 48 h.
Data collection
After careful examination of the patients' medical files, clinical data, laboratory data (collected before RBC transfusion, if required for the treatment of ACS) and radiological data were collected using a standardized electronic case report form. If laboratory data were not available for the day of ACS onset, we analysed data recorded within 24 h of onset (if available, and as long as the patient had not received an RBC transfusion). If the patient had received an RBC transfusion in the 30 days immediately before the ACS, the laboratory data were censored. Our radiological diagnosis of ACS was based on a CT scan or an X-ray of the thorax. No specific criteria were needed to perform a CT scan and this was left to the physician's discretion. Pneumococcal vaccination was considered to be effective if administered in the previous 5 years, and influenza vaccination was considered to be effective if administered in the previous 12 months. Fever was defined as a body temperature ≥38°C measured with a tympanic thermometer. Incentive spirometry is prescribed in our centre for every SCD inpatient in case of VOC, to prevent ACS. It is part of systematic inpatient prescriptions, as morphine or fluid therapy.
Each patient's medical history (as recorded in the referral centre's electronic database and collected during routine follow-up visits) was documented for the first ACS episode in the study period. Chronic complications were evaluated at steady-state, that is at least 3 months after an acute episode of VOC or ACS, as described elsewhere.21-23
Ethics
The study was approved by our local independent ethics committee (CERAPHP.5 [Comité d'Ethique pour la Recherche Assistance Publique Hôpitaux de Paris.5], 15 March 2019, reference 2019-02-04, IRB [Institutional Review Board] registration #00011928). In line with the French legislation on retrospective studies of routine clinical practice, participants were not required to give their written consent. The participants were nevertheless informed that their personal medical data could be used for research purposes and did not object to this use.
Statistical analyses
Groups were compared using an analysis of variance (for continuous variables, expressed as the median [interquartile range (IQR)]) and Fisher's exact test (for qualitative variables, expressed as the frequency [percentage]). Statistical analyses were performed with R Studio® software (version 1.2.1335; R Core Team [2016] R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [https://www.R-project.org/]). The threshold for statistical significance was set to p < 0.05.
RESULTS
Characteristics of the study population
In all, 117 ACS episodes were recorded prospectively (Figure 1). After the application of our exclusion criteria, 105 of the 117 episodes (in 81 patients) were selected for analysis. Forty-four of the 105 episodes of ACS (42%) were classified as early-onset, and 61 (58%) as secondary.
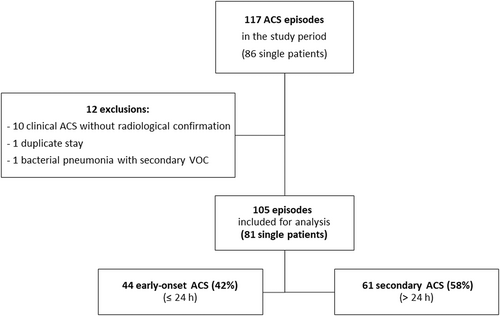
At the time of the first ACS episode, the median [IQR] age of the 81 patients was 27 [22–34] years (Table S1). There were 48 males (59%), and 72 patients (89%) had an S/S or S/β0 thalassaemia genotype. Sixty-six patients (81%) had a history of ACS, with a median of 3 [2–5] episodes per patient (of which 1 [0–2] led to ICU admission). A history of pulmonary embolism (PE) was noted for 4 patients (6%). The “early-onset” and “secondary” groups of patients did not differ significantly with regard to the prevalence of chronic complications of SCD.
Characteristics of the ACS episodes at diagnosis, as a function of time to onset
Patients had completed pneumococcal and influenza vaccinations in 70% (61/87) and 50% (34/68) of the episodes, respectively (Table 1). A disease-modifying treatment was ongoing in 61% (64/105) of the cases. Among patients on chronic therapy, 94% (60/64) were taking hydroxyurea (HU). Twenty-seven of the 105 episodes (26%, in 19 different patients) were recurrences (median of 2 [2–3] episodes (range: 2–4) per patient). Fever was present at diagnosis of ACS in 42% (37/89) of episodes (median temperature: 38.5 [38.2–38.9]°C in this subgroup). In 21% (11/52) of the episodes without fever on admission, fever was noted at least 24 h after ACS onset (median time interval: 1 [1–1.5] day). Overall, fever occurred in hospital during 54% (48/89) of the episodes. The median respiratory rate (RR) was 24 [17–30]/min, and the value was significantly higher in the secondary ACS group than in the early-onset ACS group (25 [20–35] vs. 19.5 [16–27.5], respectively; p = 0.012). For secondary ACS, the median [IQR] time to onset was 2 [2–3] days after admission. When considering severe episodes of ACS (43 of the 105 episodes [41%]), 19 (44%) featured severe tachypnea (RR >30/min). This proportion was higher for secondary ACS than for early-onset ACS (16/25 (64%) vs. 3/18 (17%), respectively; p = 0.006). Severe tachypnea was the most prevalent severity sign (Table 1 and Table S4). Bone pain was noted for all episodes of ACS. The most frequent pain sites were the sternum and/or ribs (77/105, 73%), the limbs (38/105, 36%), and the lumbar spine (35/105, 33%). The presence of sternum/rib pain was significantly more frequent in early onset ACS than in secondary ACS (37/44 (84%) vs. 40/61 (66%), respectively; p = 0.044). The most frequent abnormal auscultation finding was crackles (64%, 67/105; essentially in the lower areas and never in the uppermost areas). The crackles were bilateral in 42% (28/67) of cases. Bronchial breathing was noted for 32% (34/105) of the episodes (Table S2). The groups did not differ significantly with regard to the prevalence of physical examination signs.
| N a | All episodes N = 105 | Early-onset ACS N = 44 | Secondary ACS N = 61 | p | |
|---|---|---|---|---|---|
| Age (years) | 105 | 27 [22–34] | 27 [22–33.2] | 26 [22–34] | 0.577 |
| Male sex | 62/105 (59%) | 28/44 (64%) | 34/61 (56%) | 0.430 | |
| BMI (kg/m2) | 100 | 21.8 [19.5–23.9] | 21 [19.5–23.9] | 21.9 [19.6–23.7] | 0.867 |
| Genotype | |||||
| SS | 92/105 (88%) | 37/44 (84%) | 55/61 (90%) | 0.382 | |
| S-Beta0 thalassaemia | 3/105 (3%) | 1/44 (2%) | 2/61 (3%) | 1 | |
| SC | 9/105 (9%) | 5/44 (11%) | 4/61 (7%) | 0.487 | |
| S-O arab | 1/105 (1%) | 1/44 (2%) | 0/61 (0%) | 0.419 | |
| Pneumococcal vaccination | 61/87 (70%) | 19/34 (56%) | 42/53 (79%) | 0.030 | |
| Influenza vaccination | 34/68 (50%) | 12/25 (48%) | 22/43 (51%) | 1 | |
| Disease-modifying treatment | 64/105 (61%) | 22/44 (50%) | 42/61 (69%) | 0.068 | |
| Hydroxyurea | 60/64 (94%) | 22/22 (100%) | 38/42 (90%) | 0.289 | |
| daily dose (mg/kg/day) | 60 | 20.3 [16.5–25] | 22.5 [18.1–25.3] | 22 [16.9–25.8] | |
| Transfusion program | 6/64 (9%) | 0/22 (0%) | 6/42 (14%) | 0.086 | |
| Vital signsb | |||||
| RR (/min) | 91 | 24 [17–30] | 19.5 [16–27.5] | 25 [20–35] | 0.012 |
| RR >20/min | 59/91 (65%) | 19/38 (50%) | 40/53 (75%) | 0.015 | |
| Oxygen saturation (%) | 92 | 98 [92.8–100] | 98 [93.5–100] | 98 [91–100] | 0.516 |
| Oxygen therapy | 72/95 (76%) | 28/40 (70%) | 44/55 (80%) | 0.333 | |
| Heart rate (/min) | 90 | 99.5 [85.2–115.8] | 98 [82–113.8] | 100 [86.8–116] | 0.447 |
| Systolic BP (mm Hg) | 89 | 120 [106–134] | 121 [109–133] | 119 [105.8–135.5] | 0.946 |
| Diastolic BP (mm Hg) | 89 | 69 [60–80] | 77 [69–81] | 67 [59–75.8] | 0.026 |
| Temperature (°C) | 89 | 37.4 [36.9–38.3] | 37.3 [36.9–38.4] | 37.4 [36.8–38.2] | 0.847 |
| Fever at diagnosis | 37/89 (42%) | 13/38 (34%) | 24/51 (47%) | 0.279 | |
| Delayed-onset fever | 11/52 (21%) | 7/23 (30%) | 4/29 (14%) | 0.190 | |
| Temperature >38.5°C | 15/89 (17%) | 7/38 (18%) | 8/51 (16%) | 0.783 | |
| Bone pain | |||||
| Chest | 77/105 (73%) | 37/44 (84%) | 40/61 (66%) | 0.044 | |
| Limbs | 38/105 (36%) | 17/44 (39%) | 21/61 (34%) | 0.685 | |
| Spine | 35/105 (33%) | 18/44 (41%) | 17/61 (28%) | 0.209 | |
| Cervical spine | 3/105 (3%) | 2/44 (5%) | 1/61 (2%) | 0.570 | |
| Thoracic spine | 17/105 (16%) | 12/44 (27%) | 5/61 (8%) | 0.014 | |
| Lumbar spine | 24/105 (23%) | 11/44 (25%) | 13/61 (21%) | 0.814 | |
| Pelvis | 10/105 (10%) | 6/44 (14%) | 4/61 (7%) | 0.314 | |
| Phlegm | 12/103 (12%) | 8/44 (18%) | 4/59 (7%) | 0.119 | |
| Bright yellow phlegm | 6/12 (50%) | 2/8 (25%) | 4/4 (100%) | 0.061 | |
| Severe ACS | 43/105 (41%) | 18/44 (41%) | 25/61 (41%) | 1 | |
| RR >30/minc | 19/43 (44%) | 3/18 (17%) | 16/25 (64%) | 0.006 |
- Note: The results are expressed as the median [interquartile range] or the number (%). Statistically significant p-values are given in bold type.
- Abbreviations: BMI, body mass index; BP, blood pressure; PE, pulmonary embolism; RR, respiratory rate; VOC, vaso-occlusive crisis.
- a For continuous variables.
- b Vital parameters available at the time of ACS diagnosis.
- c Severity signs are described in detail in Table S4.
There were few significant intergroup differences in laboratory variables (Table 2). It is noteworthy that the serum C-reactive protein (CRP) was significantly higher for secondary ACS than for early-onset ACS (134.5 [97.9–197.3] vs. 35.2 [14.4–116.3] mg/L, respectively; p < 0.001).
| N a | All episodes N = 105 | Early-onset ACS N = 44 | Secondary ACS N = 61 | p | |
|---|---|---|---|---|---|
| Haemoglobin (Hb) (g/dL) | 103 | 8.2 [7–9.5] | 8.6 [7.5–9.9] | 8.1 [6.9–9] | 0.094 |
| HbS (%) | 38 | 81.8 [73.3–87.5] | 81.8 [73.8–85.1] | 84 [74.5–87.7] | 0.880 |
| HbF (%) | 38 | 8.1 [3.7–12.4] | 9 [4.2–14.7] | 7.5 [3.2–10.1] | 0.138 |
| MCV (fL) | 99 | 85 [79–90] | 84 [77–90] | 85 [80–90] | 0.401 |
| Chronic HU therapy | 60 | 87 [81.5–93] | 86 [80–93] | 87 [82–94.5] | 0.602 |
| Reticulocytes (G/L) | 94 | 204 [148.5–293.2] | 179 [135–281] | 210 [152–302] | 0.996 |
| Platelets (G/L) | 95 | 352 [253–445.5] | 359 [307–471] | 345 [244.2–433] | 0.244 |
| Leukocytes (G/L) | 98 | 14.4 [10.6–17.6] | 14.7 [10.7–19] | 14.3 [10.6–16.3] | 0.615 |
| LDH (IU/L) | 72 | 474.5 [357.5–688.2] | 522 [367–839.5] | 438 [349–606] | 0.315 |
| Total bilirubin (μmol/L) | 81 | 43 [29–63] | 43 [30–56] | 43 [28–77.2] | 0.273 |
| Conjugated bilirubin (μmol/L) | 69 | 13 [8–19] | 12 [8–18] | 13 [8.2–19] | 0.199 |
| Serum aspartate transaminase (IU/L) | 81 | 55 [35–78] | 61 [46–85] | 47.5 [30–71.2] | 0.945 |
| Serum alanine transaminase (IU/L) | 84 | 24.5 [16–45] | 30 [19–50] | 22 [14–37] | 0.231 |
| Serum alkaline phosphatase (IU/L) | 81 | 104 [75–156] | 106 [74–166] | 104 [76–148] | 0.814 |
| Serum GGT (IU/L) | 80 | 52 [28–95.2] | 69.5 [29.2–106.2] | 44 [28–86.5] | 0.267 |
| Serum CRP (mg/L) | 85 | 109.1 [32.9–155.7] | 35.2 [14.4–116.3] | 134.5 [97.9–197.3] | <0.001 |
| Serum creatinine (μmol/L) | 95 | 47 [40–58] | 49 [41.8–62.2] | 46 [38.5–56] | 0.078 |
| Estimated GFR (CKD-EPI) (mL/min/1.73 m2) | 95 | 136 [127–146] | 135.5 [126.5–145] | 137 [128.5–147.5] | 0.065 |
| Arterial blood gas | |||||
| pH | 63 | 7.4 [7.4–7.4] | 7.4 [7.4–7.4] | 7.4 [7.4–7.4] | 0.141 |
| <7.35 | 1/63 (2%) | 1/27 (4%) | 0/36 (0%) | 0.174 | |
| PaO2 (mm Hg) | 63 | 80 [68–106.5] | 86 [70–106.5] | 78 [66–96.8] | 0.320 |
| <60 | 7/63 (11%) | 2/27 (7%) | 5/36 (14%) | 0.560 | |
| PaCO2 (mm Hg) | 63 | 43 [38.5–46.5] | 41 [38–45.5] | 44.5 [39–48.2] | 0.537 |
| >50 | 7/63 (11%) | 2/27 (7%) | 5/36 (14%) | 0.244 | |
| Lactate (mmol/L) | 62 | 0.8 [0.7–1.1] | 0.8 [0.6–1.1] | 0.8 [0.7–1.1] | 0.362 |
- Note: The results are expressed as the median [interquartile range] or the number (%). Statistically significant p-values are given in bold type.
- Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CRP, C-reactive protein; GFR, glomerular filtration rate; GGT, gamma glutamyl-transpeptidase; HbF, fetal haemoglobin; HbS, haemoglobin S; HU, hydroxyurea; LDH, lactate dehydrogenase; MCV, mean corpuscular volume; PaCO2, arterial carbon dioxide partial pressure; PaO2, arterial oxygen partial pressure.
- a For continuous variables.
Positive microbiological samples were documented for 20% (21/105) of the episodes (Table S3). A single bacterial pathogen was found in 8.5% (9/105) of the episodes. Antigenurias were almost always negative. Sputum cultures were positive for 42% (17/42) of the samples, with oropharyngeal flora (10/17, 59%), Staphylococcus aureus (4/17 [24%]), Haemophilus influenzae (2/17, 12%), and Klebsiella pneumoniae (1/17, 6%). When performed, blood cultures were always sterile (0/68). The only broncho-alveolar lavage sample was positive for oropharyngeal flora. A Chlamydia pneumoniae infection (1%) was detected in a serology assay (positive for IgMs, negative for IgGs). One influenza A virus was detected on a polymerase chain reaction (PCR) test of a nasopharyngeal swab (1/10 PCR performed).
A chest X-ray was performed for 73% (77/105) of the ACS episodes and a CT scan (mostly with contrast enhancement) was performed for 60% (63/105) (Table 3). The most frequent abnormal finding was alveolar condensation (in 74/105 episodes [70%]; most cases were bilateral [77/105, 73%] and at the bottom of the lungs [71/105, 68%]). Pleural effusion was observed in 40% (42/104) of the episodes and PE was diagnosed in 3% of episodes. No difference was found between groups concerning radiological findings.
| N a | All episodes N = 105 | Early-onset ACS N = 44 | Secondary ACS N = 61 | p | |
|---|---|---|---|---|---|
| Type of imagingb | |||||
| Chest X-ray | 77/105 (73%) | 34/44 (77%) | 43/61 (70%) | 0.506 | |
| CT scan (no contrast) | 3/105 (3%) | 2/44 (5%) | 1/61 (2%) | 0.570 | |
| CT scan (with contrast) | 60/105 (57%) | 22/44 (50%) | 38/61 (62%) | 0.235 | |
| Type of infiltrate | |||||
| Alveolar | 74/105 (70%) | 29/44 (66%) | 45/61 (74%) | 0.396 | |
| Interstitial | 8/105 (8%) | 4/44 (9%) | 4/61 (7%) | 0.717 | |
| Mixed | 23/105 (22%) | 11/44 (25%) | 12/61 (20%) | 0.633 | |
| Site | |||||
| Bilateral | 77/105 (73%) | 32/44 (73%) | 45/61 (74%) | 1 | |
| Right base | 18/105 (17%) | 6/44 (14%) | 12/61 (20%) | 0.601 | |
| Left base | 16/105 (15%) | 8/44 (18%) | 8/61 (13%) | 0.584 | |
| Both bases | 71/105 (68%) | 29/44 (66%) | 42/61 (69%) | 0.834 | |
| Right apex | 2/105 (2%) | 0/44 (0%) | 2/61 (3%) | 0.508 | |
| Left apex | 5/105 (5%) | 2/44 (5%) | 3/61 (5%) | 1 | |
| Both apexes | 2/105 (2%) | 1/44 (2%) | 1/61 (2%) | 1 | |
| Extended right | 5/105 (5%) | 4/44 (9%) | 1/61 (2%) | 0.159 | |
| Extended left | 7/105 (7%) | 5/44 (11%) | 2/61 (3%) | 0.127 | |
| Number of lobes involved | 101 | 2 [2–2] | 2 [2–2] | 2 [1–2] | 0.542 |
| Pleural effusion | 42/104 (40%) | 13/43 (30%) | 29/61 (48%) | 0.105 | |
| Abundance | |||||
| Mild | 33/42 (79%) | 10/13 (77%) | 23/29 (79%) | 0.613 | |
| Moderate | 7/42 (17%) | 2/13 (15%) | 5/29 (17%) | 1 | |
| Large | 2/42 (5%) | 1/13 (8%) | 1/29 (3%) | 0.528 | |
| Site | |||||
| Right | 12/42 (29%) | 3/13 (23%) | 9/29 (31%) | 0.722 | |
| Left | 7/42 (17%) | 3/13 (23%) | 4/29 (14%) | 0.657 | |
| Bilateral | 23/42 (55%) | 7/13 (54%) | 16/29 (55%) | 1 | |
| Pulmonary embolism | 2/60 (3%) | 1/22 (5%) | 1/38 (3%) | 1 |
- Note: The results are expressed as the median [interquartile range] or the number (%).
- Abbreviation: CT, computed tomography.
- a For continuous variables.
- b At the time of diagnosis.
The median [IQR] duration of hospital stay was 9 [7–11] days, and patients were hospitalized for a median [IQR] of 7 [5–10] days after the ACS onset (Table 4). An RBC transfusion (or manual blood exchange) was made in 50% (53/105) of cases. Among severe cases, 67% (29/43) had a transfusion. Antibiotics were used in almost all episodes (96/105, 91%), cefotaxime (50/96, 52%) and spiramycin (40/50, 80%) being the most frequent. Thirty episodes (29%) led to a transfer in ICU. Non-invasive ventilation was used in 5 cases and mechanical ventilation was required for one 27-year-old S/C patient, who died of multiorgan failure with hepatic sequestration. Hence, the in-hospital mortality rate after ACS was 0.95% (1/105).
| N a | All episodes N = 105 | Early-onset ACS N = 44 | Secondary ACS N = 61 | p | |
|---|---|---|---|---|---|
| Total length of stay (days) | 105 | 9 [7–11] | 9 [6–13] | 9 [7–11] | 0.839 |
| Time from ACS onset to discharge | 105 | 7 [5–10] | 8.5 [6–12.5] | 6 [4–9] | – |
| Transfusion | 53/105 (50%) | 23/44 (52%) | 30/61 (49%) | 0.844 | |
| Number of RBC packs | 53 | 2 [2–2] | 2 [2–3] | 2 [2–2] | 0.326 |
| Severe ACS | 29/43 (67%) | 14/18 (78%) | 15/25 (60%) | 0.325 | |
| Time since diagnosis (days) | 51 | 1 [0–3] | 1.5 [0–3] | 1 [0–2] | 0.926 |
| Method | |||||
| Manual blood exchange | 27/53 (51%) | 9/23 (39%) | 18/30 (60%) | 0.170 | |
| Transfusion | 27/53 (51%) | 13/23 (57%) | 14/30 (47%) | 0.583 | |
| Erythrocytapheresis | 3/53 (6%) | 3/23 (13%) | 0/30 (0%) | 0.076 | |
| Antibiotics | 96/105 (91%) | 40/44 (91%) | 56/61 (92%) | 1 | |
| Transfer to the ICU | 30/105 (29%) | 17/44 (39%) | 13/61 (21%) | 0.079 | |
| Artificial ventilation | 6/30 (20%) | 2/17 (12%) | 4/13 (31%) | 0.360 | |
| In-hospital mortality | 1/105 (1%) | 0/44 (0%) | 1/61 (2%) | 1 |
- Note: The results are expressed as the median [interquartile range] or the number (%).
- Abbreviations: ACS, acute chest syndrome; ICU, intensive care unit; RBC, red blood cell.
- a For continuous variables.
DISCUSSION
We comprehensively described 105 consecutive ACS episodes in adult patients with SCD and compared the characteristics as a function of the time to onset after hospital admission. No major differences between early-onset and secondary episodes were found.
The French definition of ACS is slightly different from the definition of the Cooperative Study for Sickle Cell Disease (CSSCD) and National Acute Chest Syndrome Study Group (NACSSG) (new infiltrate on imaging and any of 5 clinical criteria between chest pain, fever >38.5°C, tachypnea, wheezing, or cough).24 These American criteria derive from studies mainly conducted on paediatric patients. The French definition includes detailed auscultatory abnormalities (crackles, bronchial breathing and decreased sounds) that we believe are critical in the early detection of ACS in adults and probably more accurate than chest pain, which may be due to rib VOC without lung involvement. Six episodes in our study did not perfectly match with the clinical CSSCD and NACSSG criteria, but all presented dyspnea, crackles or bronchial breathing and all had lung condensation on imaging, in the absence of other cause, making the diagnosis of ACS reliable. The British definition (fever and/or respiratory symptoms, accompanied by a new pulmonary infiltrate on chest X-ray) is even less detailed.25 An international consensus for the definition of ACS would be useful.
Fifty-eight percent of the ACS episodes were diagnosed after more than 24 h in hospital (median [IQR]: 2 [2–3] days); these values are in line with the literature data.3, 4 With a median [IQR] duration of stay of 9 [7–11] days, ACS is responsible for prolonged hospitalizations (average of 4.4 days in our centre in case of non-complicated VOC). 26 We noted a history of ACS for 81% of the patients, with a median [IQR] number of 3 [2–5] previous episodes. This is similar to the value of 76% for 128 SCD patients aged 20 or over in Vichinsky et al. US study (published in 2000) of ACS in a population of adults and children.24 In a previous study of our centre's cohort, 162/231 patients (70.1%) had a history of ACS with a median of 1 [0–3] episodes in their lifetime.23 In the present study, the proportion of patients with an S/S or S/β0-thalassaemia genotype was higher (89%) than in our previous study (79%), although the median ages were equivalent. These findings emphasize that the vaso-occlusive phenotype is more severe for S/S and S/β0-thalassaemia patients than for S/C patients, and that ACS may be a recurrent complication. Here, it is noteworthy that 19 patients experienced two or more episodes of ACS during the 2.5-year study period, highlighting the risk of subsequent ACS when a first episode has occurred. This observation should prompt physicians to carefully monitor these patients as soon as they arrive at the emergency department after a VOC. Some patients are more susceptible to ACS than others, and whether this is due to genetic or environmental factors is unclear. Moreover, secondary-onset ACS occurred despite the systematic use in our centre of incentive spirometry for every inpatient with VOC. While this study was conducted in an expert centre with careful attention to regular adaptation of disease modifying therapy to severity, only 61% of our patients were taking HU at the time of hospital admission (median [IQR] daily dose: 20.3 [16.5–25] mg/kg/day), even though this treatment is recommended in patients with a history of ACS. Ongoing disease-modifying treatment was noted only for 63% (17/27) of the recurrences. Moreover, the median mean corpuscular volume (MCV) in the 60 patients on chronic HU therapy was 87 [81.5–93] fL, and only 4 of them (7%) had a median MCV > 100 fL. These real-life data highlight the problem of patients sometimes refusing disease modifying therapy or having poor therapeutic compliance as suggested by MCV data collected in our study, despite systematic revaluation of treatment plan at discharge and proposal of therapeutic education sessions. However, our results seem better than those of the recent study by Minniti et al. in American SCD centres where 42% (28/66) of patients were on HU despite 62% (41/66) having a history of ACS.27
Overall, fever was noted at least once for 54% of the episodes; this proportion is lower than in the literature (from 64% to 81%).6, 13, 24, 28, 29 This difference might be due to the small proportion of episodes with an infectious cause. As previously discussed, the standardized definition of ACS by the CSSCD and NACSSG does not require fever as clinical sign; temperature measurement is of course important, but this cannot be used as much of a medical decision-making criterion. In contrast, the prevalence of chest pain (73%) and crackles (64%) is consistent with the literature data.6, 13, 28 Interestingly, bronchial breathing was observed in 32% of episodes; to the best of our knowledge, this phenomenon has not previously been described in the literature on ACS. Although bronchial breathing is a classical lung auscultation finding in case of pulmonary consolidation,30 it is sometimes difficult to recognize in the absence of training.31 These physical examination signs are very useful in low-resources countries (e.g. in Africa), where there is little access to chest imaging, and this clinical approach could also decrease the use of radiations in SCD patients (at least by avoiding systematic chest imaging in case of simple VOC), who are exposed from an early age.32
Twenty-one of the 105 microbiological samples (20%) were positive. Sputum samples were most frequently collected (38%, 40/105) and mostly evidenced oral flora. It should be noted that our study was performed before the coronavirus disease 2019 pandemic. Furthermore, our microbiological work-up was guided by the patient's symptoms and was not systematic; hence, the positivity rate was probably overestimated. However, our present findings are similar to those of a recent French study of 61 adult patients with SCD and febrile ACS; microbiological samples were collected in all cases, and pathogens were documented in 12 of these (19.7%).33 Our findings are also consistent with other literature reports, in which the microbial positivity rate ranged from 1% to 25%.13, 24, 34, 35 Overall, the microbiological characteristics of ACS are poorly documented, even when systematic, in-depth microbiological work-up is performed.13 In contrast, antibiotics were prescribed for 91% of the episodes of ACS: all febrile episodes and 84% (44/52) of the initially non-febrile episodes. Considering the low overall frequency of samples with a single documented bacterial species (9/105, 8.5%), these data may correspond to an overuse of antibiotics. Indeed, in adult patients with SCD, antibiotic therapy should be restricted to febrile and/or severe episodes of ACS and should be fine-tuned as a function of the bacteria detected.33 Moreover, and as emphasized by the glomerular hyperfiltration observed in our patients at the time of ACS (median [IQR] eGFR: 136 [127–146] mL/min/1.73 m2), elevated doses of antibiotic might be required to achieve a sufficient plasma concentration and thus avoid treatment failure.36 Specific clinical and pharmacokinetic studies are therefore needed to determine how best to manage empirical antibiotic therapy and optimize the corresponding dosage regimens (especially for beta-lactams), as well as randomized studies to address a key question about the indication of antibiotics in afebrile ACS, or about the duration of antibiotics in febrile and non-severe ACS.
Although a higher serum CRP concentration was significantly more frequent in secondary ACS, this result was not totally unexpected. Indeed, Veil et al. showed that a rapid increase in serum CRP (up to 60 mg/L) during the first 2 days was a normal trajectory for a non-complicated VOC.26
RBC transfusion was performed in 50% of the episodes, which is similar to other observational studies of adult patients with SCD in non-ICU hospital wards,4, 13 but lower than the proportion reported in adults by Vichinsky et al. (61%).24 Moreover, transfusion was required in 67% (29/43) of the severe cases; this proportion can be as high as 83% in the ICU.14 In France, an RBC transfusion sparing policy has been implemented for the past 10 years at least to limit iron overload and allo-immunization2; therefore, transfusions are discussed in case of ACS with severity criteria, or in patients in chronic transfusion program (indicated for various reasons, i.e. stroke, repeated VOC, multiple ACS, pulmonary arterial hypertension, etc.), countering potentially severe secondary allo-immunization and delayed hemolytic transfusion reaction. We did not note the reason of transfusion for each episode, but this transfusion policy did not appear to have an impact on mortality in our series. Indeed, in-hospital mortality rate was 0.95% (1/105), which is much lower than the value of 7% reported by Vichinsky et al.,24 or the 1.6% noted by Allareddy et al. for over 24 000 hospitalizations for ACS in the USA.12
The most frequent radiological findings in our series are consistent with the literature data.6, 13, 37 Lung CT data were available for 57% of the episodes of ACS but only 2 of the 60 (3%) evidenced PE. This prevalence of PE is lower than 12%–17% observed in two recent French studies of ICU patients with severe ACS,38, 39 although the discrepancy might be due to differences in severity. This prevalence is also lower than the value of 9.8% reported by Vichinsky et al., although the researchers did not have CT data for all episodes.24 It should be borne in mind that a causal relationship between PE and ACS (occurrence or severity) has not been clearly established. Despite these high prevalence rates (especially in ICU studies), we note that the ACS-specific mortality rate among adult with SCD has fallen over the last few decades, although PE may not always be looked for, especially in non-ICU wards, and therefore undertreated. Furthermore, ACS-specific mortality is more attributable to acute respiratory distress syndrome and multiorgan failure than to pulmonary arterial thrombosis.14 Lastly, imaging cannot show whether the PE is caused by blood clots or is linked to sickled RBC aggregates, and definitive diagnoses cannot be made for the older post-mortem studies.40, 41
The main strengths of our study were the comprehensive, time-of-onset-based description of a large series of consecutive ACS episodes in adult patients with SCD and the homogeneous application of diagnostic criteria in a national SCD referral centre (previous prospective studies of ACS, especially those performed in emergency departments (which can complicate data collection and interpretation), might have included a small, selected subgroup of the potentially eligible patients). In contrast, retrospective collection (with missing data) and the absence of comparison of ACS episodes as a function of the time since the start of the VOC (which was not part of our dataset collection) constitute study limitations.
CONCLUSION
We found that most cases of ACS occurred a few days after hospital admission for a VOC but that it was sometimes already present on admission. Secondary and early-onset forms of ACS do not appear to differ significantly. Disease-modifying treatments should be revaluated after each ACS episode because the recurrence rate is high. Moreover, in order to counter the rise in antibiotic resistance, evidence-based antimicrobial stewardship and optimized dosage regimens are needed; although fever is frequent in ACS, infectious triggers are rare and the prognosis is good.
AUTHOR CONTRIBUTIONS
G. Cheminet and J.-B. Arlet designed the study and wrote the manuscript. G. Cheminet and D. Khimoud collected the data. A. Brunetti and A.-S. Jannot performed the analyses. J.-B. Arlet, B. Ranque, J. Pouchot, A. Michon and E. Flamarion cared for patients. All authors contributed to data interpretation, revised the manuscript for critical content and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank all the practitioners in our unit for their commitment to caring for patients with SCD. No specific funding was needed for this study.
CONFLICT OF INTEREST STATEMENT
None of the authors report any conflicts of interest with regard to the present study.




