Low B-cell content is associated with a CD73-low tumour microenvironment and unfavourable prognosis in classic Hodgkin lymphoma
Johanna Grund, Katharina Iben and Sarah Reinke contributed equally.
Summary
B-cell content in the tumour microenvironment (TME) of classic Hodgkin lymphoma (HL) is known to be associated with prognosis. Here we demonstrate that whole slide image analysis using routinely available slides predicts outcomes in patients treated with ABVD in a prospective trial with a high B-cell content being associated with a favourable prognosis. B cells in the TME did not correlate with B cells in peripheral blood. In the TME maturation, stages of B cells (naive and memory) were consistent. However, we detected down-regulation of CD73 in HL with low B cells suggestive of an antibody-independent function of B cells in the TME of HL.
INTRODUCTION
Classical Hodgkin lymphoma (HL) is characterized by an abundant tumour microenvironment (TME) which is considered crucial for survival and proliferation of the malignant Hodgkin and Reed-Sternberg cells (HRSC). T cells are the most abundant population in the TME and their role in the pathogenesis of HL is increasingly understood.1-3 B cells comprise only about 16% of all cells in the TME by whole slide image analysis (WSI) but the content varies from case to case. Multiple studies have identified low B-cell content in the TME to be associated with unfavourable prognosis of HL using different methods of B-cell quantification.4-7 Using WSI, we recently confirmed the prognostic relevance of B-cell content in advanced-stage HL treated with bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone (eBEACOPP). However, several questions regarding the clinical relevance and the pathogenetic role of B cells in the TME of HL remain unanswered so far. For example, whether WSI is able to predict outcomes in HL treated with doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) has not yet been investigated. Moreover, data on the phenotype of B cells in the TME of HL are limited preventing any conclusion regarding their pathogenetic role.
METHODS, RESULTS AND DISCUSSION
We analysed primary tumour biopsies of patients with newly diagnosed early stage unfavourable HL either treated with 4 × ABVD or 2 × eBEACOPP followed by 2 × ABVD (‘2 × 2’) within the German Hodgkin Study Group (GHSG) HD14 trial, each consolidated by 30 Gy involved-field radiotherapy.8 Our project cohort (N = 198) was carefully selected as previously described9 to match the HD14 trial population (N = 1889, Table S1) but additionally enriched for patients suffering from disease progression or relapse (20% vs. 6% in the total HD14 population). WSI of CD20 staining was performed by TissueStudio 64 (Definiens) to quantify the B-cell content as previously described (Figure 1A, Supplementary Material).9
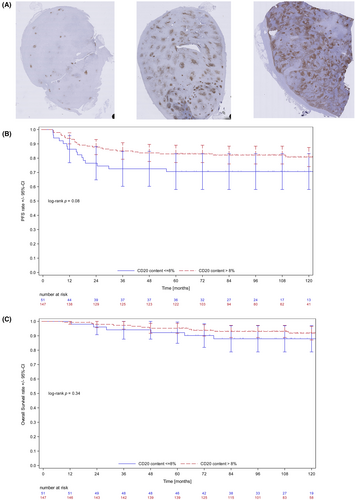
To investigate the prognostic value of B-cell content, a multivariate analysis of progression-free survival (PFS) was performed which included treatment arm, age, sex, clinical stage, presence of B-symptoms and the four clinical GHSG-risk factors. Backward selection of variables with p < 0.1 was used (Table S2). CD20 content was the only significant factor in the resulting model (p = 0.0196, OR = 0.95, 95% CI: 0.9–0.99). With an optimal cut-off determined at 8% by a receiver-operating characteristic (ROC) analysis (Table S3), there was a trend towards unfavourable outcomes with low B-cell content: (5-year PFS estimates of 71% for subjects with low (≤8%) and 83% with high (>8%) B-cell content, respectively (Figure 1B, log-rank p = 0.08). The differences in PFS did not translate into significant differences in overall survival (OS) (Figure 1C). When patients were split according to treatment the cut-off of 8% performed descriptively better in the group with 4 x ABVD compared to the group with 2 × eBEACOPP followed by 2 × ABVD. However, in this small subgroup analysis, the log-rank test was not significant (Figure S1). Nevertheless, we conclude that WSI of CD20 to determine low B-cell content might be a universal tool for prognostication of HL outcome, widely available by simply reanalysing routinely available immunohistochemistry stainings. However, the most relevant cut-off varies between different risk groups analysed (10% and 21% in two previously analysed cohorts of advanced-stage HL9 and 8% in the current study of early stage unfavourable HL). It is not unexpected that a prognostic biomarker in HL depends on the clinical context exemplified by gene expression-based classifiers which differ strikingly according to therapeutic conditions.7, 10
We correlated B-cell content in the TME assessed by WSI in HL patients treated in the NIVAHL trial11 with pretreatment B-cell content in the peripheral blood.12 B-cell content in the TME of HL did not correlate with B-cell content in the peripheral blood as relative to all CD45+ cells or absolute B-cell counts/μL (Figure S2A,B respectively). As reported previously,13 focusing on the peripheral blood only, relative blood overall B-cell content was positively correlated with naïve and inversely correlated with memory B cells (Figure S2C,D).
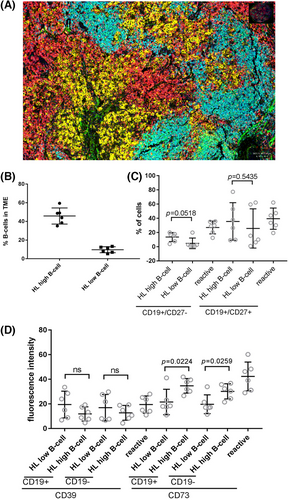
Next, we analysed n = 12 HL specimens using fluorescence multi-staining and quantitative image analysis by QuPath software (Müller-Meinhard, in preparation) to gain insight into B-cell phenotypes associated with high and low B-cell content in the TME. The staining panel was composed of CD19, CD27, CD30, CD39 and CD73. Cases were selected based on material availability and B-cell content to represent a high (35%–59%) and low (5%–13%) B-cell content (n = 12 with n = 6 in each group, Figure 2B). WSI captures the complete B-cell content in a tissue biopsy including regressive but potentially pre-existing B-cell follicles.9 To analyse the TME in a narrower sense, we selected areas with the distance to a neoplastic HRSC (identified as CD30 positive cell) was a maximum of 25 μm (Figure 2A). The absence of germinal centres (GC) is a generally acknowledged feature of the TME of most HL. We thus aimed to understand if content in naïve and memory B cells differ according to overall B-cell content and quantified CD27 expression on CD19-positive B cells. The TME of HL contains fewer CD19+/CD27− naïve B cells than interfollicular areas of reactive lymph nodes (n = 6) independent of the overall B-cell content by WSI (p = 0.0140 and p = 0.0009 for the group with high and low B-cell content, respectively, Figure 2B). Memory B-cell content (CD19+/CD27+) did not differ between either groups of HL and reactive tissue (Figure 2B). There was a higher content of naïve B cells in the group of HL with high compared to low B-cell content, which did not reach statistical significance (p = 0.0518, Figure 2B). Since maturation/differentiation of B cells does not seem to differ between HL with a relative B-cell-rich and a B-cell-depleted TME, we analysed antibody-independent functions of B cells by assessing the expression of CD39 and CD73 on B cells. Both proteins contribute to an immune-modulatory milieu through hydrolyzing exogenous adenosine triphosphate (ATP) to adenosine 5′-monophosphate (AMP) by CD39 and subsequently AMP to adenosine (ADO) by CD73.14 The effects of AMP and ADO on B and T cells are complex but AMP is generally considered to inhibit and ADO to induce T-cell proliferation.14 We did not detect any difference in a relative number of CD39 or CD73 positive B cells in the two groups of HL with high and low B-cell content respectively (data not shown). Similarly, expression of CD39 and CD73 bulk mRNA did not differ between the two groups, whereas CD20 (MS4A1) gene expression differed as expected (Figure S3). However, expression by fluorescence intensity was significantly lower for CD73 in the group of HL with low compared to high B-cell content (p = 0.0224, Figure 2C). This difference was also detectable when a normalization with CD27 as an unrelated protein intensity was performed to exclude tissue variability of staining (Figure S3C). Moreover, the CD73 intensity in HL with low B-cell content was significantly reduced in the B cells compared to B cells in the interfollicular region of reactive lymph nodes (p = 0.0085, Figure 2C) while the intensity of CD73 in the group of HL with high B-cell content did not differ from B cells in reactive tissue (p = 0.9998, Figure 2C). In contrast, the expression intensity of CD39 (Figure 2C) and CD27 (data not shown) was not different between groups of HL with high and low B-cell content and not significantly different from B cells in reactive tissue (Figure 2C, p = 0.1833). Of note, the reduced expression of CD73 was not only confined to B cells but also detected in CD19-negative cells (p = 0.0259, Figure 2D). Our data indicate a dysbalanced expression of CD39 and CD73 confined to the group of HL with B-cell-depleted TME. Reduced levels of CD73 could potentially lead to an accumulation of immunosuppressive AMP in the intercellular compartment of the HL. Intracellular levels of ATP, AMP and ADO are about 1000 times higher than in the intercellular compartment. Consequently, differences in levels of these metabolites in bulk tissue analysis were not detected by metabolite analysis (Figure S3D).
In summary, we confirm the potential prognostic impact of a reduced overall B-cell content in the TME of HL determined by WSI also early stage unfavourable HL and with different types of polychemotherapy (ABVD and eBEACOPP). Due to its wide applicability, B-cell content might represent one of the best TME-derived prognostic markers in HL. Moreover, we provide first insights into B-cell phenotypes in the HL TME, including a potential pathomechanism underlying an unfavourable clinical HL course when B-cell content is low. Our data generated on a limited set of samples certainly warrant confirmation but also suggest that B-cell composition with respect to maturation stages (naïve and memory) does not differ between high and low B-cell-containing TME. Instead, we identified the down-regulation of CD73 as a potential mechanism creating an immunosuppressive milieu, in line with previous observations.2 Yet, down-regulation of CD73 is not a phenomenon restricted to B cells representing an example of the global immunomodulatory effect of HL on the immune landscape, as also illustrated in peripheral lymphocytes.2, 12 So far, it is still uncertain whether accumulations of B cells in the TME of HL represent actors (tumour-associated tertiary lymphoid structures) or bystander cells (remnants of pre-existing follicles).5 Additionally, the role of B-cell content and phenotypes in light of immune-checkpoint inhibition remains to be explored.
AUTHOR CONTRIBUTIONS
Johanna Grund, Katharina Iben, Sarah Reinke, Fabian Kellermeier, Berit Müller-Meinhard, Maria A. Garcia Marquez and Hans A. Schlößer performed the research. Wolfram Klapper designed the research study. Bastian von Tresckow, Peter Borchmann, Annette Plütschow and Peter Borchmann contributed essential reagents or tools. Johanna Grund, Sarah Reinke, Wolfram Klapper, Ina Bühnen and Annette Plütschow analysed the data. Wolfram Klapper wrote the paper. All authors read and agreed on the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors thank all patients enrolled in the GHSG clinical trials, all clinicians and pathologists supporting the clinical trials and the translational research program of the GHSG. We thank Charlotte Botz-von Drathen, Dana Germer and Lorena Valles Uriarte for excellent technical support. Scanning and image analysis technology was supported by the Kinderkrebsinitiative Buchholz, Holm-Seppensen (KKI). We thank Katja Dettmer-Wilde and Peter Oeffner for supporting AMP analysis and Ina Bühnen for supporting statistical analysis. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
Scanning and image analysis technology was supported by the Kinderkrebsinitiative Buchholz, Holm-Seppensen (KKI).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no specific disclosures for the present study.
PATIENT CONSENT STATEMENT
Patients consented to a translational analysis upon registration.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
n.a.
CLINICAL TRIAL REGISTRATION (INCLUDING TRIAL NUMBER)
n.a.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




