Combining MYD88 L265P mutation detection and clonality determination on CSF cellular and cell-free DNA improves diagnosis of primary CNS lymphoma
Clotilde Bravetti and Michaël Degaud contributed equally to this work.
Summary
Diagnosis of primary central nervous system lymphoma (PCNSL) is challenging, and although brain biopsy remains the gold standard, cerebrospinal fluid (CSF) constitutes a less invasive source of lymphomatous biomarkers. In a retrospective cohort of 54 PCNSL cases tested at diagnosis or relapse, we evaluated the contribution of immunoglobulin heavy chain (IGH) gene clonality and MYD88 L265P detection on both CSF cell pellets and supernatants, in comparison with cytology, flow cytometry, interleukin (IL)-10 and IL-6 quantification. Clonality assessment included a new assay to detect partial IGH-DJ rearrangements. Clonal IGH rearrangements and/or MYD88 L265P mutation were detected in 27 (50%) cell pellets and 24 (44%) supernatant cell-free (cf) DNA. Combining analyses on both compartments, 36 (66%) cases had at least one detectable molecular marker, present only in cfDNA for 9 (16%) of them. While cytology and flow cytometry were positive in only 7 (13.0%) and 9 (17.3%) cases respectively, high IL-10 levels were observed in 36 (66.7%) cases. Overall, taking into account molecular and cytokine results, 46/54 (85%) cases had at least one lymphomatous biomarker detectable in the CSF. These results show that this combination of biomarkers evaluated on both cell pellet and supernatant CSF fractions improves significantly the biological diagnosis of PCNSL.
Abbreviations
-
- CBA
-
- cytometric bead array
-
- cfDNA
-
- cell-free DNA
-
- CSF
-
- cerebrospinal fluid
-
- ctDNA
-
- circulating tumor DNA
-
- ddPCR
-
- digital droplet polymerase chain reaction
-
- DLBCL
-
- diffuse large B-cell lymphoma
-
- FCM
-
- flow cytometry
-
- FR
-
- framework regions
-
- IG
-
- immunoglobulin
-
- IGH
-
- immunoglobulin heavy chain
-
- IL
-
- interleukins
-
- LOC
-
- lymphomes oculo-cerebraux
-
- NGS
-
- next-generation sequencing
-
- PCNSL
-
- primary central nervous system lymphoma
-
- PCR
-
- polymerase chain reaction
-
- RFLP
-
- restriction fragment length polymorphism
-
- SHM
-
- somatic hypermutation
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is a rare type of lymphoma whose diagnosis must be rapidly performed but remains difficult.1-3 The localization is mainly cerebral but may also involve the spinal cord, leptomeninges or the eye. The vast majority are diffuse large B-cell lymphoma (DLBCL).4 The tumour cells are thought to derive from late germinal centre B cells arrested in their terminal differentiation and display highly mutated immunoglobulin (IG) genes.5 The genetic landscape of PCNSL is characterized by recurrent mutations of MYD88 and CD79B genes,6-10 the most frequent being the MYD88 L265P hotspot mutation with a prevalence of 60% in a large meta-analysis study.11
The diagnosis of PCNSL is based on a combined assessment of clinical features, magnetic resonance imaging and detection of tumour cells in the cerebrospinal fluid (CSF) or vitreous fluid using cytology and flow cytometry. Molecular techniques on cells collected from CSF constitute additional tools improving the diagnosis. Detection of clonal IG rearrangements is a well-recognized methodology for assessing the malignant character of lymphoid B-cell populations,12 but the reported sensitivity in the CSF of PCNSL is rather low,13-15 partly explained by the high frequency of somatic hypermutations (SHM). The MYD88 L265P hotspot mutation is also an attractive but not constant target.16, 17 The benefit of these molecular assays is somehow hampered by the small quantity or the absence of tumour cells present in the CSF. Indeed, the tumour cells in PCNSL are often in small quantity or absent thus leading to frequent negative results.18 Quantification of cytokines such as interleukin-10 (IL-10) and IL-6 has been shown to provide useful complementary biomarkers: an increased IL-10 CSF level and IL-10 /IL-6 ratio allow to discriminate PCNSL from other neurologic diseases,19, 20 independent or not of the presence of tumour cells. The definitive diagnosis, however, relies ultimately on a brain biopsy1, 21 but depending on its localization, the tumour might be too difficult to access or the biopsy not contributive. In addition, it is an invasive procedure with possible surgical complications.22
Recently, the ability to detect circulating tumour DNA (ctDNA) in the CSF has attracted much attention for the diagnosis of brain tumours including lymphomas,16, 17, 23-29 as it constitutes a less invasive alternative. In fact, CSF appears much more informative than plasma for such purpose.27 The vast majority of studies have focused on oncogenic alterations using single16, 17, 23, 25, 26 or multiple target9, 28, 29 identification. However, only very few have considered IG gene rearrangements,30 although they constitute genomic markers present in all lymphoid B cells.
In this study, we aimed at evaluating the contribution of molecular analysis including clonality and MYD88 L265P detection on CSF cell pellets and supernatants, in comparison with other techniques used in the standard diagnosis of PCNSL (cytology, flow cytometry and cytokine quantification). In addition, we developed a new assay to detect partial IGH gene rearrangements, a well-suited target for short size DNA fragments and devoid of SHM.12, 31
MATERIALS AND METHODS
Patients
Fifty-four patients with confirmed diagnosis of PCNSL followed at the Pitié-Salpêtrière and Institut Curie—René Huguenin hospitals during a 2-year period (2015–2016) were selected for this retrospective study (Table S1). They were part of the French oculo-cerebral lymphoma (LOC) registry.32, 33 Most of them were part of a larger cohort previously published.18 The study was approved by the local ethics review committee, and informed consent was obtained from the patients.
Sample collection
CSF was obtained from lumbar puncture at diagnosis (n = 41) or relapse (n = 13) and collected in several tubes, either without additive or containing the Transfix® cell stabilizing agent for flow cytometry (FCM) evaluation. Processing time from sampling was variable but did not exceed 24 h. All cases underwent cytomorphologic examination of May–Grunwald–Giemsa-stained cytospins performed by experienced haematologists. Additional information is provided in the Data S1.
Flow cytometry
CSF cells were labelled with an eight-colour FCM antibody panel including anti-CD45 (HV500, Becton Dickinson), CD3 (BV421, Becton Dickinson™), CD4 (PE-CY7, Becton Dickinson™), CD8 (APC-AF750, Beckman Coulter), CD5 (APC, Becton Dickinson™), CD19 (PC5.5, Beckman Coulter™), kappa (FITC, Agilent™) and lambda (PE, Agilent™) and analysed on a FACSCanto II cytometer (BD Biosciences™) as previously described.18 Data were analysed with Becton Dickinson FACSDiva software. Depending on the number of events with restricted surface light chain expression, samples were classified as positive (cluster of 20 events or more), negative (cluster of less than 10 events) or inconclusive (cluster of 10–19 events).
Cytokine quantification
IL-10 and IL-6 were quantified using the cytometric bead array (CBA) technique (human IL-10 and IL-6 CBA kits; BD Biosciences) according to the manufacturer's recommendations. The detection limit had been determined at 2.5 pg/mL and a positive threshold above 4 pg/mL.19, 20 A sample with IL-10 > 4 pg/mL and/or an IL-10:IL-6 ratio >1 was considered as positive.18 It was considered negative when IL-10 was <2.5 pg/mL. In other situations, the results were considered as inconclusive.
DNA extraction
CSF samples were centrifuged (16 000 g, 10 min) and supernatants were stored at −80°C until further analysis. Cell pellets were resuspended in 60 μL of lysis buffer containing 0.45% Tween 20 (Sigma-Aldrich), 0.45% Igepal CA-630 (Sigma-Aldrich), PCR buffer (Life Technologies) and proteinase K (10 mg/mL, Eurobio Scientific) and incubated for 1 h at 56°C followed by 10 min at 100°C. The lysates were spun down and stored at −20°C until further processing. Cell-free DNA was extracted from 400 µL to 1800 μL (mean 1091 ± 341 μL) CSF supernatants using the QIAamp Circulating Nucleic Acid kit (Qiagen) according to the manufacturer's instructions. Quantification (ng/μL) was determined by converting results (copy number/μL) from digital droplet polymerase chain reaction (ddPCR) experiments (Data S1).
Clonality
Lymphoid clonality was assessed by amplification of both complete (VDJ) and partial (DJ) immunoglobulin heavy (IGH) chain gene rearrangements followed by capillary electrophoresis of PCR products (GeneScan analysis).12 Further details are provided in the Data S1.
Detection of MYD88 L265P mutation
Different methods were used for the detection of MYD88 mutation (c.794T>C, p.L265P) according to the type of DNA. On cell pellet DNA, an in-house restriction fragment length polymorphism (RFLP) assay based on previous publications34, 35 was used. Alternatively, on cfDNA this mutation was detected by ddPCR, using the QX200™ analyser (Bio-Rad). Further details on primers, probes and reagents are provided in the Data S1.
RESULTS
Molecular marker detection on cell pellets
We assessed B cell clonality on the cell pellet DNA extracted from 54 CSF samples from PCNSL patients (Figure 1). We first searched for complete IGH-VDJ rearrangements using Biomed-2 protocols12 and detected a clonal rearrangement in 9/54 (17%) cases. Using the assay for incomplete IGH-DJ rearrangements, a clonal peak was found in nine samples, seven of which had no detectable IGH-VDJ rearrangement. Combining both complete and incomplete IGH rearrangements, a monoclonal B-cell population was detected in 16/54 (30%) cases.
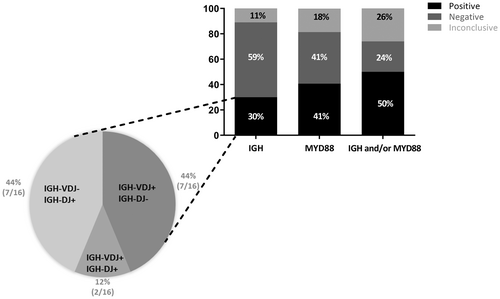
Using the PCR-RFLP technique, we then searched for the presence of the MYD88 L265P mutation which was detected in 22/54 (41%) samples (Figure 1). Among the mutated cases, 11/22 (50%) had no detectable IGH clonal rearrangement (Tables S2 and S3). Combining clonality and MYD88 L265P mutation, a molecular marker was detected in cell pellet DNA for half of the patients (27/54, 50%).
Molecular marker detection on supernatants
We next performed molecular assays on cfDNA extracted from the CSF supernatants (Figure 2). Because of the small size of cfDNA fragments, only FR3 PCR was performed for analysis of complete IGH-VDJ rearrangements. A clonal rearrangement was detected in 11/54 (20%) cases. Due to the low amount of DNA obtained from supernatants, only 35 samples could be evaluated for IGH-DJ clonality, 6 (17%) of which were found to be clonal. Among them, two had no detectable IGH-VDJ rearrangement. A clonal IGH-VDJ and/or IGH-DJ rearrangement was therefore observed in 13/54 (24%) cases, although a fraction could not be tested for IGH-DJ clonality (19/54).
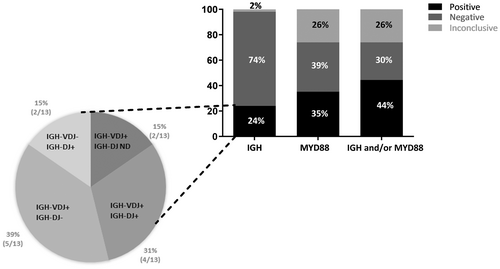
For the detection of MYD88 L265P mutation, as the amount of cfDNA from CSF supernatant was expected to be low, we used a technique known to be more sensitive than the one employed for cell pellets, that is ddPCR.36 In addition, it allowed us to calculate the amount of cfDNA extracted from CSF supernatant (see Material and Methods section), as well as the allelic ratio of the L265P mutation. Thirty-five out of 54 samples contained a total amount of cfDNA lower than 1 ng. Using ddPCR, we found the mutation in cfDNA for 19/54 (35%) patients. The mutation ratio ranged from 9% to 93%, with six samples having a mutation ratio >50% (data not shown). Among the mutated samples, 11/19 (58%) had no detectable IGH clonal rearrangement. Taking together IGH clonality and MYD88 L265P mutation, 24/54 (44%) cases had at least one positive molecular marker detectable in the supernatant (Tables S2 and S3).
Comparison of molecular markers on both compartments
We next compared molecular results obtained from cell pellets and supernatants. Among the 54 samples, 36 (67%) had at least one detectable molecular marker. Twenty-three (43%) showed a clonal IGH rearrangement (Figure 3A). It was detected in both compartments in 6/54 (11%) cases, only in the cell pellet in 10/54 (19%) cases and only in the supernatant in 7/54 (13%) cases.

More than half of the samples (28/54, 52%) were positive for the MYD88 L265P mutation (Figure 3B). It was detected in both cell pellet and supernatant in 13/54 (24%) cases, only in the cell pellet in 9/54 (16.7%) cases, and only in the supernatant in 6/54 (11%) cases.
We next focused on the 27/54 (50%) cases for which molecular techniques on cell pellet were either negative (n = 15) or inconclusive (n = 12) in order to evaluate the contribution of cfDNA analysis. Importantly, a third of them (9/27 33%) had at least one molecular marker positive on the supernatant (Figure 3C).
Comparison of molecular markers with FCM and cytokine quantification
Cytological examination was performed systematically but only seven cases displayed evidence of lymphomatous cells in the CSF, all these cases being positive for at least one other biomarker. FCM results were available for 52 patients and positive in 9 (17%) of them (Figure 4A). For each of these nine patients, a molecular assay was also found to be positive, either on cell pellet (n = 3), on supernatant (n = 1) or in both compartments (n = 5). Regarding the 43 patients for whom FCM was negative or not contributive, at least one molecular assay was positive for almost two-thirds of them (27/43, 63%). For 10/43 (23%) cases a molecular marker was detected on both cell pellet DNA and cfDNA, only on cell pellet for 9/43 (21%) cases, and interestingly only on supernatant for 8/43 (19%) cases.
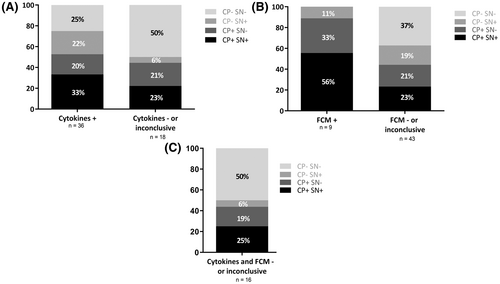
Cytokine quantification (IL-10 and IL-6) was consistent with the diagnosis of PCNSL for 36/54 (67%) samples (Figure 4B and Table S4). Twenty-seven of them (75%) had also at least one detectable molecular marker, either on the cell pellet (7/36, 19%), the supernatant (8/36, 22%) or on both compartments (12/36, 33%). For the 18 patients with negative or inconclusive cytokine quantification results, half of them (9/18, 50%) had at least one molecular marker positive either on cell pellet (4/18, 22%), on supernatant (1/18, 6%) or in both compartments (4/18, 22%).
Focusing on the 16 patients for whom both FCM and cytokine quantification results were negative or inconclusive, half of them (8/16, 50%) had at least one molecular assay positive (Figure 4C). A molecular marker was detected in both cell pellet and cfDNA in 4/16 (25%) cases, cell pellet only in 3/16 (19%) cases and cfDNA only in 1/16 (6%) cases. Taken together, these results show that molecular assays can be positive even when both FCM and cytokine quantification are negative.
Finally, in only 8/54 (15%) patients all techniques failed to detect evidence of the lymphomatous population in the CSF. All results are summarized in Table S2.
DISCUSSION
The diagnosis of PCNSL is often challenging and although the formal proof requires pathology examination of a tumour biopsy, analysis of CSF can be of considerable help. Several recent reports have shown that in addition to the direct detection of lymphomatous cells, the finding of ctDNA in the CSF may bring indirect evidence for the presence of tumour cells in the CNS.16, 17, 23-30 In this study, we have used several techniques on both the cellular fraction and the supernatant of CSF to better identify less invasive biomarkers for PCNSL diagnosis.
All CSF samples were first analysed by FCM, which has the advantage of providing rapid assessment of the presence of lymphomatous cells. However, leptomeningeal dissemination is an inconstant finding in PCNSL.15, 37, 38 In addition, failure to detect lymphomatous cells may include lack of sensitivity of the technique, cell mortality and treatment of patient with corticoids before lumbar puncture.39
Demonstration of clonal rearrangement of antigen receptor genes is a well-established method for assessing the neoplastic nature of a lymphoid population.12 However, when applied to CSF rather low levels of success have been reported.40-42 This may be due in part to the absence or the small number of circulating tumour cells.43 Another source of failure for amplifying IGH gene rearrangements comes from the fact that most PCNSL IG variable region genes harbour a high level of SHM.44-46 Partial IGH-DJ rearrangements represent good alternative targets as they are devoid of such mutations12, 31 but are an inconstant feature, as shown by their detection in only 40% of systemic DLBCL.47 Here, we used a modified set of IGH-DJ primers, generating smaller and much more homogeneous size of PCR products thereby simplifying result interpretation.48 This new assay is well-adapted to small size DNA fragments and increases significantly the chance of detecting clonal IGH rearrangements in these B-cell tumours with highly mutated IG genes.
A meta-analysis of 40 studies including 2736 patients with DLBCL revealed a prevalence of the MYD88 L265P mutation in 60% of those occurring in the PCNSL.11 In our cohort, the detection rate in cell pellet DNA was 41%, while a recent study found higher (72%) or similar (41%) rates in newly diagnosed and relapsed PCNSL, respectively.16 The differences might be explained, at least in part, by differences in volumes of CSF collected and technical aspects. Indeed, we used a simple, rapid and cost-effective PCR-RFLP method, initially developed to identify this mutation on blood or bone marrow samples for the diagnosis of Waldenström macroglobulinaemia.35 This proved adequate for incompletely purified DNA obtained by rapid lysis extraction of CSF cell pellets. Importantly, half of the MYD88 L265P-mutated cases had no detectable clonal IGH rearrangements, pointing out the complementary value of using these two molecular markers.
As leptomeningeal invasion is a relatively infrequent feature of brain tumours, detection of ctDNA in the CSF has emerged as an attractive minimally invasive diagnosis strategy.49 In fact, ctDNA has been found much more frequently in the CSF than in the blood for these tumours, reflecting localized shedding of tumour DNA by dying cells.28 The yield of DNA recovery from the CSF supernatants in our study was fairly low, being less than 1 ng in slightly less than two-thirds of cases. This may be due in part to the delay between lumbar puncture and sample processing. Cell-free DNA is known for being prone to rapid degradation and CSF processing should be performed in less than 6 h and ideally within 2 h after sampling.26, 50 Unfortunately, in this retrospective study based on ‘real life’ routine diagnostic laboratory practice, such considerations had not been taken into account for all cases, with a delay ranging from 1 to 24 h before CSF reception. In addition, the low DNA yield may also be due to the retrospective nature of this study as Hiemcke-Jiwa et al.25 reported better results on fresh CSF samples as compared to frozen ones. Furthermore, the amount of CSF collected for molecular studies was limited with a majority of cases having less than 1 mL. It is therefore expected that with additional material and reduced processing time, better results might be obtained.
That notwithstanding, clonal IGH rearrangements were found in almost a quarter of supernatants, including additional cases not detected in the cell pellets. To our knowledge, this is the first time that conventional lymphoid clonality is performed on CSF cfDNA. Due to the limited quantity of cfDNA retrieved from CSF supernatants, we employed a more sensitive technique (ddPCR) for MYD88 L265P mutation detection than the one used on cell pellets. As for IGH, additional mutated cases not seen on cell pellets were detected on supernatants. Therefore, cfDNA is a complementary source of molecular markers for the diagnosis of PCNSL. In addition, it might also serve to monitor treatment response and predict relapse.24, 28
Cytokines such as IL-10 are soluble markers released by lymphomatous cells which might not circulate in the CSF, and as such are somewhat comparable to ctDNA. IL-10 (and IL-10:IL-6 ratio) was in fact the biomarker with the highest rate of positivity in our cohort, and for one quarter of all cases it was the only detectable biomarker. Failure to detect IL-10 secretion might be due to the use of steroids prior to CSF collection which are known to inhibit cytokine production.19, 39 Importantly, half of the cases with negative or inconclusive cytokine results had a molecular marker in the cell pellet and/or the supernatant, illustrating their complementary diagnosis value.
Several recent studies have reported that high-throughput sequencing of selected gene panels (or even whole exome) is feasible on cfDNA obtained from CSF and can identify tumour-associated mutations in primary or metastatic brain tumours as well as primary or secondary CNS lymphoma.24, 51, 52 Using highly sensitive next-generation sequencing (NGS) techniques, Mutter et al. have reported very high rate of ctDNA detection in the CSF samples of patients with PCNSL.53 Similarly, detection of clonal IG rearrangement by a NGS assay has been recently proposed as a useful tool for brain lymphoma diagnosis.30 However, these methods require more DNA, more expertise and are more expensive. They also necessitate time to perform analyses and interpret data, which may delay therapy initiation. In contrast, our approach is based on conventional and well-established methodologies, and is thereby easily applicable in a large number of diagnostic laboratories. Furthermore, testing of CSF is often used to rule out the diagnosis of a putative CNS lymphoma. Thus, fairly simple, rapid and robust screening tests may be more adapted.
In conclusion, our study shows that combining several biomarkers as well as using both cellular and extra-cellular CSF material improves significantly the diagnosis of PCNSL. We confirm that CSF supernatant is a useful source of ctDNA and efforts should be made in clinical and laboratory practice to optimize yield and quality of such material.
AUTHOR CONTRIBUTIONS
Clotilde Bravetti and Michaël Degaud designed and performed the experiments, analysed the data, prepared the figures and wrote the paper. Marine Armand, Elise Sourdeau, Jennifer Osman and Patricia Verrier performed the experiments and analysed the data. Karim Maloum performed cytology. Karim Maloum performed histopathology analyses. Magali Le Garff-Tavernier and Elise Sourdeau supervised flow cytometry investigations including cytokine quantification. Caroline Houillier, Damien Roos-Weil, Sylvain Choquet and Khe Hoang-Xuan were responsible for patient care and provided patient data. Jérôme Alexandre Denis designed and analysed the ddPCR experiments. Frédéric Davi designed the study, analysed the data and wrote the paper. All authors reviewed and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
This work was supported in part by an ERA-NET TRANSCAN-2/French National Cancer Institute grant (NOVEL consortium), and by Cancer United Research Associating Medicine, University and Society (CURAMUS), INCA-DGOS-Inserm_12560.
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests.




