Efficacy of haematopoietic stem cell boost as a rescue for poor graft function after haematopoietic stem cell transplantation: A multicentre retrospective study on behalf of the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC)
S. Pagliuca and C. Pochon are co-last authors.
[Correction added on 12 April 2023, after first online publication: The article title was corrected in this version.]
Summary
Haematopoietic stem cell reinjection may be a curative option for poor graft function after haematopoietic stem cell transplantation; however, literature supporting its use remains limited. We conducted a multicentre retrospective study on behalf of the Francophone Society of Bone Marrow Transplantation and Cellular Therapy, including 55 patients. We demonstrated response rates of nearly 40% and two-year survival of more than 60% in the context of an otherwise deadly complication and we observed that the timing of injection and the degree of cytopenia are strongly associated with outcomes. This study shows the feasibility of the procedure informing on its epidemiology, outcomes and prognostic factors, setting the stage for future guidelines.
Abbreviations
-
- ALL
-
- acute lymphoblastic leukaemia
-
- AML
-
- acute myeloid leukaemia
-
- BM
-
- bone marrow
-
- BMF
-
- bone marrow failure
-
- CI
-
- confidence interval (or) cumulative incidence
-
- CR
-
- complete response
-
- GF
-
- growth factor
-
- GVHD
-
- graft-versus-host disease
-
- HLH
-
- haemophagocytic lymphohistiocytosis
-
- HSC
-
- haematopoietic stem cell
-
- HSCT
-
- haematopoietic stem cell transplantation
-
- ID
-
- immune deficiency
-
- IQR
-
- interquartile range
-
- MDS
-
- myelodysplastic syndrome
-
- MPN
-
- myeloproliferative neoplasm
-
- NR
-
- no response
-
- OR
-
- odds ratio
-
- OS
-
- overall survival
-
- PB
-
- peripheral blood
-
- PGF
-
- poor graft function
-
- PR
-
- partial response
Poor graft function (PGF) is an important cause of morbidity and mortality after allogeneic haematopoietic stem cell transplantation (HSCT) affecting 5%–20% of transplanted patients.1, 2 This condition is defined by the presence of one or more long-lasting cytopenias despite the persistence of haematopoiesis of donor origin.1, 3-8 The physiopathology of PGF is poorly understood, and supposed to be mediated by elevated levels of inflammatory cytokines concomitantly to a decrease of bone marrow (BM) CD34+ cells.9-11 Haematopoietic stem cell (HSC) reinjection (boost) from the initial donor, with or without ex vivo manipulation may be a curative treatment12-15 allowing haematological recovery. However, limited knowledge is currently available regarding outcomes, toxicities and factors influencing the response to this cellular treatment.
Here, exploiting a large cohort of patients undergoing allogeneic HSCT in France, we conducted a retrospective multicentre study on behalf of the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC) to investigate outcomes and tolerance of HSC reinjections for patients with PGF. All first HSCT paediatric and adult recipients receiving a boost from July 2013 to September 2019, identified in French centres and providing written consent to participate in research protocols, were included in this study (Supporting Information). The study procedure complied with French regulatory requirements and the research was conducted according to the declaration of Helsinki.
PGF-defining cytopenias were identified as one of the following: neutrophil count less than 0.5 × 109/L, platelets less than 30 × 109/L or platelet or red blood cell transfusion dependence, for two consecutive weeks after day +28 post transplant in the absence of relapsed/persistent haematologic disorder, incomplete (<90%) donor chimerism, active infectious diseases, or drug-related myelosuppression, according to previous definitions.1, 3-8
In the absence of standard criteria to characterize haematological recovery after boost, we arbitrarily defined complete response (CR) to the procedure as the establishment of transfusion independency in previously transfused patients, together with platelets count above 50 × 109/L, haemoglobin level above 100 g/L, and neutrophil counts above 1.5 × 109/L. Partial response (PR) was defined as any improvement of the baseline counts not sufficient to reach criteria of CR and associated with transfusion independency. No response (NR) was defined by the absence of any haematological recovery as per the above description.
We identified 55 patients from 12 French centres: 28 children, five adolescents/young adults and 22 adults, with a median age of 14 years (range 0–66). Thirty-one (56%) patients were transplanted for a non-malignant condition (mainly immune deficiencies and bone marrow failure disorders), while other diagnoses included myeloproliferative neoplasms (n = 10, 18%), acute leukaemias (n = 8, 15%), myelodysplastic syndromes (n = 2, 4%) and lymphoproliferative disorders (n = 4, 7%) respectively (Figure 1A). Sixteen patients were transplanted from a matched related, 15 from a haploidentical, 14 from a matched unrelated and 10 from a mismatched unrelated donor. Eleven patients had a major ABO mismatch (Table S1).
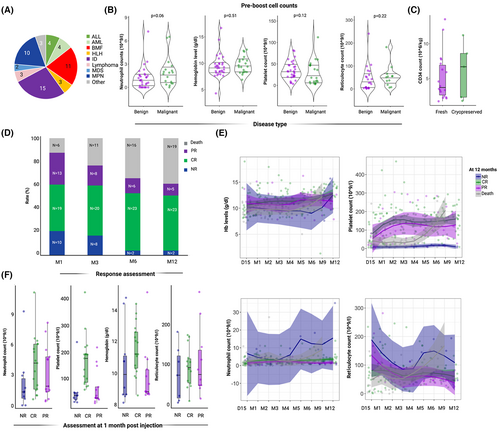
A single cellular product was administered to each patient, a CD34+-selected boost to 43 patients, and to nine patients, in a complete BM (n = 1), or pre-boost total peripheral blood (PB) graft (n = 8), reinjected without any prior conditioning regimen (missing data for three patients).
Median time of boost injection from transplant was 119 days [interquartile range (IQR): 78–222]. At the time of injection, median cell counts were 1.2 × 109/L (IQR: 0.38–1.9) for neutrophils and 31 × 109/L (IQR: 11–47) for platelets while median haemoglobin level was 93 g/L (IQR: 83–103), without any differences between patients with malignant or benign conditions (Figure 1B; Figure S1). Overall, cytopenia affected a single lineage in 16 (32%) cases, two lineages in 23 (47%) and the whole haematopoiesis in 10 patients (20%) (missing data in six patients). Notwithstanding, none of the patients with ABO mismatch developed isolated erythroblastopenia.
Pre-boost total PB or BM chimerism was complete donor in 49 cases, and mixed (<95% donor cells) in six patients. All patients with malignant disease were in complete remission before boost injection.
Pre-boost treatments included granulocytic colony-stimulating factor in 35 patients, erythropoietin in eight patients, and thrombopoietin analogues in 16 patients.
At reinjection, median cell number was 5.91 × 106 CD34/kg (IQR: 3.22–9.15) and 3.14 × 103 CD3/kg (IQR: 0–62.5). Among patients with available data, 25 received a fresh and nine received a cryopreserved stem cell boost. No difference in the number of CD34+ cells at the injection was observed between fresh and cryopreserved products and CD34+ number are reported after thawing (Figure 1C).
Of 48 evaluable patients at one month, 19 (39%) reached a CR, 13 (27%) a PR while 16 (33%) did not achieve any response. At three months CR and PR rates were respectively 43% (n = 20/47) and 17% (n = 8/47), while 40% (n = 19/47) did not achieve a response. At six months, 48% (n = 23/47) patients were in CR, 13% (n = 6/47) in PR and 38% (n = 18/47) in failure. Overall, at 12 months rates of CR, PR and failure (NR and deaths) were respectively: 47% (n = 23/49), 10% (n = 5/49) and 43% (n = 21/49) (Figure 1D).
Through univariable and multivariable general logistic regression models, we analysed which pre-boost covariates were associated with haematological response at one month post injections (Figure S2; Table S3). In this model, timing of the boost from transplant [odds ratio (OR): 1.03, 95% confidence interval (CI) 1.01–1.06, p = 0.03] and both neutrophil (OR: 5.24, 95% CI, 1.77–30.1, p = 0.014) and platelet counts (OR: 1.08, 95% CI, 1.01–1.21, p = 0.049) at the time of injection, were associated with a better response. Neither CD34+ richness, nor age, type of disease or donor impacted on the haematological response (Figure S2A).
Patients in CR at one month post injection had median counts of 4.1 × 109/L (IQR: 1.6–6.1) for neutrophils, 175 × 109/L (IQR: 85–192) for platelets, 106 g/L for haemoglobin (IQR: 88–122) and 92.5 × 109/L (IQR: 52–113) for reticulocytes (Figure 1E,F). Overall, 20 patients (36%) received at least a single-lineage growth factor (GF) as rescue post boost treatment. In multivariable models, no lineage-specific GF administration after boost injection was associated with late responses at 12 months (Figure S2B).
With a median follow-up of 788 days post boost (95% CI, 643–1132), two-year OS after injection was 60.7% (95% CI, 49.3–72.1) (Figure 2A). Cumulative incidence of de novo acute graft-versus-host disease (GVHD) at day 100 after boost was 3.6 (95% CI, 0.7–11.2), while probability of de novo chronic GVHD at one year after the procedure was 15.2 (95% CI, 7–26.3) (Figure S3A,B).
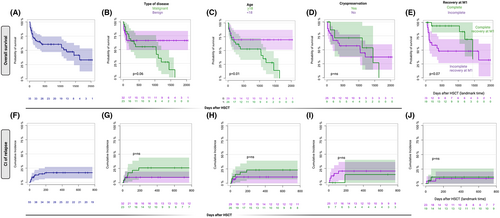
Twenty-six patients died during follow-up with a median time of 103 days (IQR 44.75–379) after boost. Causes of death were infections (n = 16), relapse (n = 6), sinusoidal obstructive syndrome (n = 2), respiratory distress (n = 1) and encephalopathy (n = 1). All patients who died from infections were not in CR at one month.
Patients with benign disorders tended to have a better OS than malignant conditions [66% (95% CI, 52–88) vs 55% (95% CI, 37–72) p = 0.06; Figure 2B]. Paediatric patients also experienced a better two-year-OS than adults (respectively 69%, 95% CI, 54–84 vs 57%; 95% CI, 41–73, p = 0.01; Figure 2C). No difference in OS were observed whether fresh or cryopreserved products were injected (Figure 2D). A slightly higher probability of survival was observed in patients who experienced CR at one month after boost, although a log-rank test was not significant (landmark analysis), likely due to the small sample size (Figure 2E).
Multivariable Cox regression analysis of pre-boost relevant clinical variables showed that male gender was the only variable positively associated with OS (hazard ratio: 0.26, 95% CI, 0.11–0.71, p = 0.0072) (Table S2).
Cumulative incidence of relapse at one year was 17% (95% CI, 8.3–28.4), while non-relapse mortality in this subset was 23.7% (95% CI, 12.9–36.3). Type of disease, age, sex, cryopreservation and complete recovery at one month (landmark analysis) were not associated with relapse probability (Figure 2F–J).
Our study shows that donor-derived HSC reinjections can be a valuable strategy to restore haematopoiesis in dysfunctional grafts, illustrating that time from transplant and degree of cytopenias may be fundamental predictors of response. In the absence of prospective clinical trials, these retrospective data characterize, so far, one of the largest cohorts published of patients with PGF receiving HSC boosts while reporting on response rates, failure and factors predicting haematological recovery.
AUTHOR CONTRIBUTIONS
M. Gaffet wrote the study protocol, followed patient recruitment and data management, interpreted the analysis, and wrote the first draft of the manuscript. A. Wiedemann helped in the statistical analysis. J.-H. Dalle, K. Bilger, E. Forcade, M. Robin, J. Cornillon, H. Labussière-Wallet, P. Ceballos, C.-E. Bulabois, M. Loschi, C. Orvain, M. T. Rubio and B. Neven contributed to patient management and data collection. S. Pagliuca performed the statistical analysis and data visualization and wrote the manuscript. C. Pochon supervised the submission of the study protocol and data collection and curation, interpreted the results, and wrote the manuscript.
ACKNOWLEDGEMENTS
The authors thank all the participating centres, with a special thanks to Nicole Raus who was very helpful in data collection and management, and the SFGM-TC scientific committee for providing support and data management time for this study. No funding was provided for the study.
CONFLICT OF INTEREST STATEMENT
This research was conducted in absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
PATIENT CONSENT STATEMENT
All participants provided written consent to participate in research protocols.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
N/A.
CLINICAL TRIAL REGISTRATION
N/A.
Open Research
DATA AVAILABILITY STATEMENT
All the data that support the findings of this study are available within the Article and Supporting Information. Patient level clinical and biological data are the intellectual property of SFGM-TC and of the centres involved in the study but can be requested from the SFGM-TC scientific committee and data management team ([email protected]).




