Addendum to British Society for Haematology Guideline for the management of mantle cell lymphoma, 2018 (Br. J. Haematol. 2018; 182: 46–62): Risk assessment of potential CAR T candidates receiving a covalent Bruton tyrosine kinase inhibitor for relapsed/refractory disease
The BSH guideline for the management of mantle cell lymphoma (MCL)1 is under review. Pending full revision of the document, the advent of chimeric antigen receptor (CAR) T-cell therapy at third-line has prompted this addendum, the focus of which is to provide additional guidance for haematologists on the selection, investigation and surveillance of MCL patients considered potential candidates for future CAR T-cell therapy.
The management of patients with MCL who progress or are intolerant to a covalent Bruton tyrosine kinase inhibitor (cBTKi) remains a significant clinical challenge.2 Tecartus, an autologous CD19-targeting CAR T-cell therapy, has been granted conditional marketing authorisation by the European Medicines Agency (EMA) for relapsed or refractory MCL after two lines of therapy, including a BTKi. The ZUMA-2 study reported impressive initial responses (overall response 93%, complete response 67%) with 37% of evaluable patients in ongoing response at a median follow-up of 35.6 months.3, 4 Overall initial responses in high-risk disease such as pleomorphic/blastoid morphology, TP53 mutations or Ki-67 proliferation index ≥50% appeared comparable but small numbers preclude valid conclusions. Significant adverse events of grade 3 or higher included cytokine release syndrome (15%), neurological events (31%) and infection (32%).3 Real-world reporting, enriched for patients with poor prognostic features, has demonstrated similar initial rates of response and toxicity.5, 6
Subsequent to approval by the National Institute for Health and Care Excellence (NICE) in February 2021, 12 centres across the UK deliver this therapy with a review of each case in England and Wales by the National CAR T Clinical Panel (NCCP) using uniform criteria (Table 1). MCL patients managed in the UK in the pre-CAR T-cell therapy era and progressing on ibrutinib represented a poor prognostic group with a post-ibrutinib median overall survival (OS) of 1.4 months for all patients and 0.4 months for those unable to receive further systemic therapy (57%).7 The latter, with a predominance of older patients, inferior Eastern Cooperative Oncology Group (ECOG) performance status (PS) and blastoid histology (32%), had achieved a median median progression-free survival (PFS) of only 3.4 months with ibrutinib, highlighting a subset with resistant and rapidly progressive disease.7 An additional pooled trial analysis and extended follow-up of 370 patients receiving ibrutinib monotherapy at relapse established that disease bulk of 5 cm or larger, raised lactate dehydrogenase (LDH), high-risk Mantle Cell Lymphoma International Prognostic Index (MIPI) score, progression of disease within 24 months of front-line therapy (POD24) and blastoid histology predict for shorter PFS and OS.8, 9 Patients from this same cohort harbouring a TP53 mutation also demonstrate poor outcomes, with a median PFS of only 4.0 months.10 Overall, the above combined population of high-risk patients represent approximately 1/3 of all MCL patients receiving ibrutinib. A significant proportion within these described cohorts will now fulfil eligibility for Tecartus.
| NHS England NCCP eligibility criteria | Comments | |
|---|---|---|
| Diagnosis | MCL with t(11;14) or cyclin D1 overexpression | |
| Age | No upper age limit | Suitability at discretion of CAR T-cell centre |
| Previous treatment |
|
Prior allograft is not an exclusion |
| Patient |
|
A prior history of MCL in the CNS is not an exclusion Medical co-morbidities at discretion of CAR T-cell centre |
| Organ function requirementsa | ||
|---|---|---|
| ZUMA-2 eligibility | Real-world practice | |
| Creatinine clearance ≥60 mls/min | >30–40 mls/min considered | Dependent on aetiology, fitness and other risk factors |
| EF ≥ 50% | EF < 50% considered | Dependent on aetiology, fitness and other risk factors |
| Oxygen saturations >92%, no pleural effusion |
Pleural effusion and ascites not an exclusion |
|
|
Bone marrow function: Platelets ≥75 × 109/l Neutrophils ≥1 × 109/l Lymphocytes ≥0.1 × 109/l |
Lower acceptable, particularly if confirmed bone marrow involvement with MCL |
|
- Abbreviations: BTKi, Bruton tyrosine kinase inhibitor; CIBMTR, Center for International Blood and Marrow Transplant Research; ECOG, Eastern Cooperative Oncology Group; EF, ejection fraction; MCL, mantle cell lymphoma.
- a Supported by real-world axi-cel CIBMTR data.16
The advent of Tecartus as third-line therapy, with the potential for durable remissions in eligible patients, warrants a review of our national approach of the investigation and surveillance of MCL at first relapse, with the goal of anticipating and capturing early refractory disease or progression on second-line ibrutinib in potential CAR T candidates with high-risk disease. Accumulating unpublished real-world UK experience with Tecartus demonstrates that pace and burden of disease are the key factors associated with failure of CAR-eligible patients reaching T-cell harvest and/or CAR T-cell infusion. A higher rate of drop-out has been observed in patients with inferior ECOG PS, blastoid histology and bulk larger than 5 cm. Such disease characteristics mirror those associated with shorter responses on ibrutinib, again reflective of a particularly poor prognostic group. With the requisite time delays built into CAR T-cell delivery (referral to a CAR centre, T-cell harvest and manufacture) CAR T-cell return can take up to eight weeks. This raises the question of the feasibility of CAR T-cell therapy in those with high-risk MCL and florid progression on second-line therapy. Theoretically, earlier referral at the first sign of ibrutinib failure may mitigate some of the risk of drop-out, improving the accessibility of CAR T-cell therapy to such patients.
We recommend that all patients considered potential candidates for future CAR T-cell therapy are assessed for cBTKi failure risk and discussed with a CAR T-cell centre at first relapse according to that risk. Referral for second-line CAR T could be considered in the context of a clinical trial. Risk assessment pre-cBTKi should include up-to-date imaging and Simplified MIPI (sMIPI) status. If patients relapse with a lymphocytosis, peripheral blood should be analysed for TP53 mutations. Where practical, a repeat biopsy should be sought to assess Ki-67 proliferation index, blastoid transformation and TP53 mutation analysis if blood sampling is not possible. This information, constitutional symptoms and disease burden should be used to formulate an impression of risk of early failure of cBTKi. High-risk patients should be followed closely; at least four-weekly face-to-face appointments in the first three months. Patients with significant constitutional symptoms showing no improvement after four weeks of ibrutinib should be considered for early re-imaging. All high-risk patients should have first imaging response assessment no later than 12 weeks. Best response of stable or progressive disease should prompt an urgent referral to a CAR T-cell centre. A repeat biopsy at progression post-BTKi should not be necessary unless imaging findings are inconclusive or an alternative diagnosis is suspected. Abrupt cessation of ibrutinib at this stage should be avoided due to risk of tumour flare.11 Stabilisation of disease may be required prior to T-cell harvest and where possible, bendamustine should be omitted due to its potential impact on T-cell fitness.4 A proposed surveillance strategy is demonstrated in Figure 1.
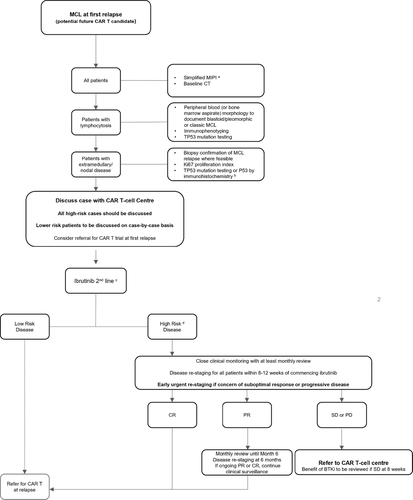
Strong predictors of long-term durable remission post CAR T-cell therapy in MCL are incompletely explored. The evidence to date is limited by small numbers and short follow-up. In a real-world dataset (n = 33), low-risk smIPI score was associated with a superior PFS.5 In high-grade B-cell non-Hodgkin lymphoma markers of disease activity (i.e., bulk, LDH), 3+ extranodal sites and inferior ECOG PS correlate with inferior survival and immediate CAR T-related toxicity post-infusion.12-15 Extrapolating this experience to MCL, adequate disease control pre-CAR T infusion may be critical to improve the drop-out rate but also to optimise the chances of durable remission and improve tolerability of Tecartus.
Recommendations
- MCL patients considered potential candidates for future CAR T treatment should be risk-assessed at first relapse prior to initiation of a BTKi. All high-risk cases should be discussed with a CAR T-cell centre.
- Assessment should include computed tomography (CT) re-staging, Simplified MIPI score with blood/tissue biopsy to establish morphology, Ki-67 proliferation index and TP53 mutation status (if feasible).
- High-risk patients starting ibrutinib should have CT re-staging within 8–12 weeks (earlier if concern).
- Lack of early response with stable/progressive disease on ibrutinib should prompt an urgent referral to a CAR T-cell centre.
AUTHOR CONTRIBUTIONS
All authors contributed to the preparation and appraisal of this manuscript. Maeve A. O'Reilly and Toby A. Eyre led on the concept and design of this document. All authors approved the final submitted version. The authors would like to thank Dr Andrew McMillan for his contribution to this work.
FUNDING INFORMATION
No funding received.
CONFLICT OF INTEREST
Maeve A. O'Reilly: advisory boards, travel and honoraria from Kite/Gilead and Novartis. Robin Sanderson: Novartis and Kite/Gilead: advisory boards, honoraria, travel. William Wilson: no conflict of interest. Sunil Iyengar: Abbvie: conference support; Beigene: advisory board; Janssen: speaker fees; Kite: advisory board, speaker fees; Takeda: advisory board, speaker fees, conference support. Jonathan Lambert: Kite: advisory boards, education, conference fees; BMS/Takeda: conference fees. Rory McCulloch: Janssen: honorarium. Toby A. Eyre: Roche: education honorarium, advisory board honorarium, travel; Gilead/Kite: Honorarium; Research support; advisory board; Janssen: Honorarium; Abbvie: honorarium; travel. AstraZeneca: honorarium, research funding, travel; Loxo Oncology: advisory board honorarium, trial steering committee; Beigene: advisory board honorarium, research funding; Incyte: advisory board honorarium; Secura Bio: advisory board honorarium.
PATIENT CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.