Arsenic trioxide inhibits anaplastic lymphoma kinase (ALK)-positive diffuse large B-cell lymphoma through targeting ALK-fusion oncoprotein
Anaplastic lymphoma kinase (ALK) gene rearrangement and chimeric nucleophosmin (NPM)-ALK fusion oncoprotein were first identified in anaplastic large cell lymphoma (ALCL).1 Fusion of ALK to other genes have subsequently been found in other neoplasms, including clathrin heavy chain 1 (CLTC-ALK) in diffuse large B-cell lymphoma (DLBCL) and echinoderm microtubule associated protein like 4 (EML4-ALK) in non-small-cell lung carcinoma (NSCLC).2, 3 ALK-positive DLBCL is uncommon but aggressive, accounting for <1% of all DLBCLs, with <100 cases reported since its first description in 1997.4 ALK-positive DLBCL have an inferior clinical outcome when treated with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or CHOP-derived regimens.5 CLTC-ALK is the most common genetic fusion in ALK-positive DLBCL, arising from t(2;17) and resulting in the chimeric oncoprotein CLTC-ALK with a constitutively activated ALK kinase domain.6 A selective ALK kinase inhibitor suppressed the growth of CLTC-ALK-positive DLBCL cells in vitro and in vivo, indicating CLTC-ALK to be a potential therapeutic target in ALK-positive DLBCL.7 Arsenic trioxide (As2O3) exerts anti-leukaemic activity by targeting the promyelocytic leukaemia/retinoic acid receptor alpha (PML-RARA) oncoprotein for degradation in acute promyelocytic leukaemia (APL). It also induces degradation of the mutant cytoplasmic NPM, NPMc+, in acute myeloid leukaemia (AML).8 We therefore proposed that As2O3 might also target NPM-ALK in ALCL. Previous work in our group has shown that As2O3 induces degradation of NPM-ALK fusion protein in ALK-positive ALCL in vitro and in vivo, thereby suppressing tumour growth of ALK-positive ALCL.9 Here we further hypothesised that As2O3 might also exhibit anti-lymphoma effect in ALK-positive DLBCL by targeting the CLTC-ALK fusion protein.
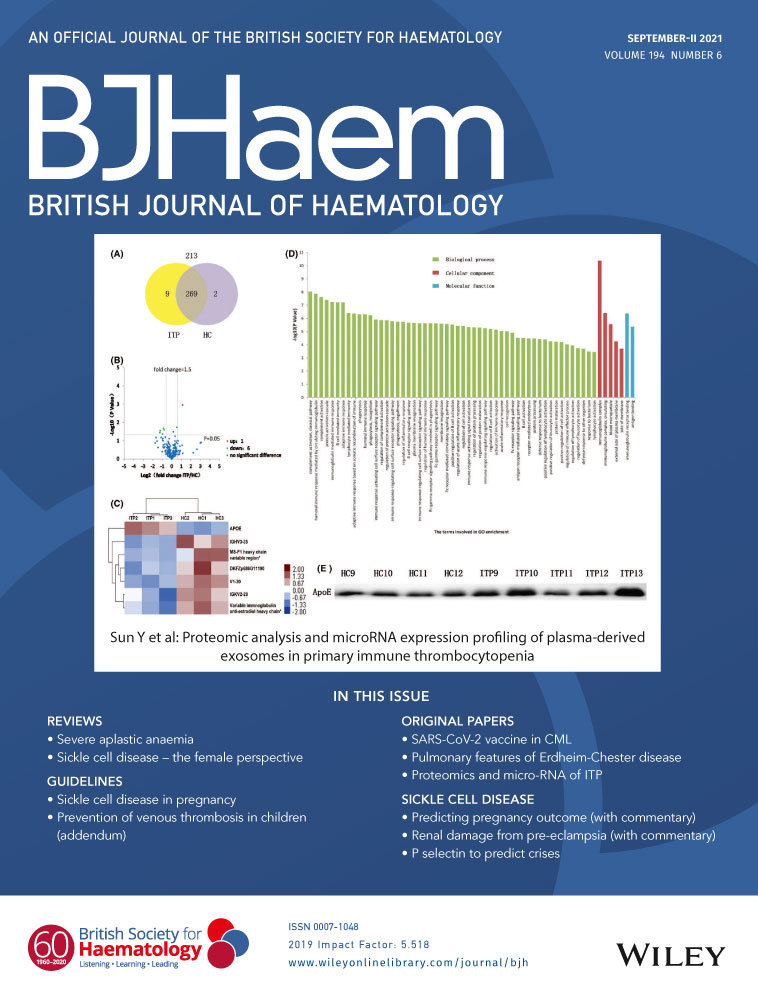
LM1 is an ALK-positive DLBCL cell line expressing the CLTC-ALK oncoprotein.7 To examine the anti-lymphoma effect of As2O3 on ALK-positive DLBCL cells, LM1 cells were treated with varying concentrations of As2O3 for 48 h, followed by cell viability assay. As2O3 treatment resulted in a dose-dependent reduction of viability of LM1 cells, where the concentration causing 50% cell growth inhibition (GI50) lies within clinical achievable concentration.10 The inhibitory activity of As2O3 was comparable with that of the selective ALK kinase inhibitor TAE684 (Fig 1A). Apoptosis assay confirmed that As2O3 induced apoptotic cell death as effectively as TAE484 (Fig 1B). These findings indicated that As2O3 exerted anti-proliferative and pro-apoptotic activities in CLTC-ALK-positive DLBCL. To further evaluate the in vivo efficacy of As2O3 in ALK-positive DLBCL, severe combined immunodeficiency (SCID)-Beige mice xenografted with LM1 line were treated with vehicle (control) or As2O3 at 5 mg/kg/day for 3 weeks, a dose regimen that has been reported for xenograft mouse model of APL.11 As2O3 treatment significantly suppressed tumour growth (Fig 1C). Furthermore, immunohistochemical staining of Ki-67 and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick-end labelling (TUNEL) assay in the excised tumours showed that As2O3 treatment inhibited cell proliferation and induced apoptosis (Fig 1D). These data suggested that As2O3 exhibited potent anti-lymphoma effect in vivo.
The effects of As2O3 on the CLTC-ALK fusion protein and ALK downstream pathways were next investigated. As2O3 treatment resulted in a dose-dependent downregulation of the CLTC-ALK protein (Fig 1E). CLTC-ALK mRNA level was unaffected, suggesting that As2O3 targeted CLTC-ALK at the post-translational level (Figure S1). Importantly, As2O3 suppressed the phosphorylation of ALK pathway downstream mediators signal transducer and activator of transcription 3 (STAT3) and protein kinase B (AKT), at a level comparable with TAE684 treatment (Fig 1F). This downregulation of CLTC-ALK protein and its downstream pathway signalling was validated in vivo. Immunohistochemical staining and Western blotting of the excised xenograft tumours showed that As2O3 treatment significantly suppressed CLTC-ALK and phosphor-STAT3 (Fig 1G; Figure S2). Overall, these findings indicated that As2O3 downregulated CLTC-ALK at the protein level, leading to suppression of ALK downstream pathway signalling. Interestingly, combination treatment of As2O3 and TAE684 significantly inhibited cell viability as compared with As2O3 or TAE684 alone across a wide range of drug concentrations in LM1 cells. Synergy studies were analysed with a computerised combination index (CI), and synergism (CI < 0·9) between As2O3 and TAE684 was observed in LM1 cells at different effective doses (ED) (Figure S3).
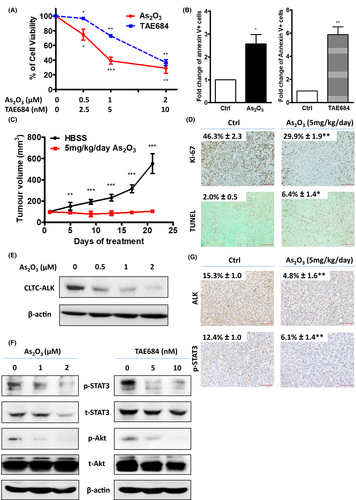
To investigate if the downregulation of CLTC-ALK was mediated by proteasomal protein degradation, LM1 cells were pre-treated with the proteasome inhibitor MG-132 prior to As2O3 treatment. Western blotting showed that MG-132 pre-treatment abrogated the As2O3-induced CLTC-ALK downregulation (Fig 2A). Furthermore, co-immunoprecipitation assay demonstrated that As2O3 enhanced the ubiquitination of CLTC-ALK (Fig 2B). Our data suggested that As2O3 targeted CLTC-ALK fusion protein for proteasomal degradation by facilitating its ubiquitination.
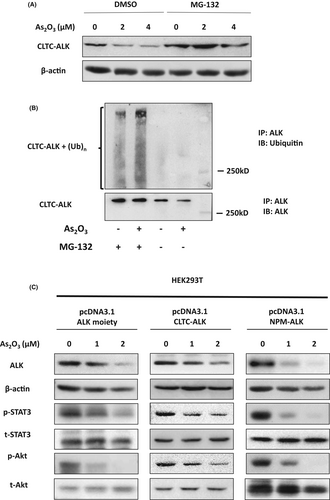
As2O3 targets another ALK fusion protein NPM-ALK for degradation in ALCL.9 As NPM-ALK and CLTC-ALK fusion proteins have the same C-terminal structure; the effect of As2O3 on the ALK moiety was investigated. Treatment with As2O3 resulted in downregulation of ectopically expressed ALK, CLTC-ALK and NPM-ALK in human embryonic kidney 293T (HEK293T) cells. More importantly, As2O3 suppressed the ALK downstream signalling pathway that was activated by the enforced expression of ALK, CLTC-ALK and NPM-ALK (Fig 2C). On the other hand, As2O3 treatment had no effect on CLTC, corresponding to the N-terminal of CLTC-ALK, (Figure S4). These findings indicated that As2O3 induced ALK fusion proteins degradation by targeting ALK instead of its fusion partners.
Previous studies have shown that As2O3 targets PML-RARA for degradation by directly binding to the cysteine residues in the PML domain, leading to the recruitment of E3 ligase RING finger protein 4 (RNF4).12 Interestingly, there are two pairs of closely spaced cysteine residues in the ALK moiety of ALK fusion protein, which are potential arsenic-binding sites. Further studies are necessary to determine if As2O3 binds to these cysteine residues directly to initiate ALK fusion protein degradation. Apart from CLTC-ALK in ALK-positive DLBCL, other ALK fusion proteins are oncogenic drivers and potential targets for treatment in different malignancies, such as EML4-ALK in NSCLC2 and tropomyosin 3 (TPM3)-ALK in inflammatory myofibroblastic tumours (IMT).13 In the present study, we showed that As2O3 targeted the ALK moiety of CLTC-ALK fusion, suggesting that As2O3 might mediate the degradation of ALK fusion proteins in general. It would be worthy and clinically relevant to study whether As2O3 also exerts anti-neoplastic effects in these malignancies through targeting the corresponding ALK fusion proteins. Studies have reported that ALK inhibition by ALK kinase inhibitors produces only short-term clinical responses in ALK-positive DLBCL cases.14, 15 These findings suggest that ALK-positive DLBCLs may develop drug resistance to ALK inhibitors. Different from ALK kinase inhibitors, As2O3 suppresses ALK oncogenic signalling through degrading the ALK fusion proteins instead of inhibiting ALK kinase activity, and hence As2O3 may potentially overcome the acquired resistance to ALK kinase inhibitors in ALK-positive malignancies.
In conclusion, CLTC-ALK is a therapeutic target of As2O3, and As2O3 treatment represents a novel therapeutic approach for ALK-positive DLBCL. Moreover, As2O3 targets the ALK moiety of ALK fusion protein, suggesting that As2O3 may be a potential therapeutic agent for other ALK-positive malignancies.
Financial Support
This study was partly supported by a General Research Fund from the Hong Kong Research Grant Council to Eric Tse.
Acknowledgments
We thank Dr Cerchietti, Cornell University, New York, USA, for the LM1 cell line.
Author contributions
Lok-Man Yue designed and performed the experiments, and wrote and approved the manuscript. David Chau performed the experiments and approved the manuscript. Yok-Lam Kwong conceived the study and approved the manuscript. Eric Tse conceived the study, designed the experiments, and wrote and approved the manuscript.
Conflict of interest
The University of Hong Kong holds the patent for the use of oral formulation of arsenic trioxide. Lok-Man Yue, David Chau, Yok-Lam Kwong and Eric Tse are employees of the University of Hong Kong.