Successful tyrosine kinase inhibitor discontinuation outside clinical trials — data from the population-based Swedish chronic myeloid leukaemia registry
Summary
Clinical trials show that tyrosine kinase inhibitor (TKI) treatment can be discontinued in selected patients with chronic myeloid leukaemia (CML). Although updated CML guidelines support such procedure in clinical routine, data on TKI stopping outside clinical trials are limited. In this retrospective study utilising the Swedish CML registry, we examined TKI discontinuation in a population-based setting. Out of 584 patients diagnosed with chronic-phase CML (CML-CP) in 2007–2012, 548 had evaluable information on TKI discontinuation. With a median follow-up of nine years from diagnosis, 128 (23%) discontinued TKI therapy (≥1 month) due to achieving a DMR (deep molecular response) and 107 (20%) due to other causes (adverse events, allogeneic stem cell transplant, pregnancy, etc). Among those stopping in DMR, 49% re-initiated TKI treatment (median time to restart 4·8 months). In all, 38 patients stopped TKI within a clinical study and 90 outside a study. After 24 months 41·1% of patients discontinuing outside a study had re-initiated TKI treatment. TKI treatment duration pre-stop was longer and proportion treated with second-generation TKI slightly higher outside studies, conceivably affecting the clinical outcome. In summary we show that TKI discontinuation in CML in clinical practice is common and feasible and may be just as successful as when performed within a clinical trial.
Introduction
With the introduction of imatinib, and subsequently second and third generation tyrosine kinase inhibitors (TKI), the survival of patients with chronic myeloid leukaemia (CML) in the chronic phase (CP) has improved dramatically and is now approaching that of healthy age-matched controls.1, 2 After TKI initiation a rapid decline in BCR-ABL1 transcripts, as measured by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), is typically seen, and with several years of continued treatment a majority of the patients will obtain a ‘deep molecular response’ [DMR, defined as a BCR-ABL1 value of <0·01% (molecular response, MR4) according to the International scale (IS)].3 Initially considered to be a lifelong treatment, several TKI discontinuation trials have shown that approximately 40–50% of patients with sustained DMR can successfully discontinue TKI and attain a treatment-free remission (TFR).4-7 Detectable but stable levels of BCR-ABL1 are seen in a proportion of these patients, challenging the previous idea that leukaemic stem cells need to be eradicated in order to maintain a durable remission.8, 9 This has shifted the initial definition of molecular recurrence from any detection of the BCR-ABL1 transcript (as in the STIM1 study) to loss of major molecular response (MMR: MR3; as in most subsequent discontinuation trials).4, 5, 10 In the largest discontinuation trial to date, the EURO-SKI, the vast majority of relapses occurred during the first six months after discontinuation.4 Although treatment with second-generation TKI, as compared with imatinib, has been shown to induce faster and deeper molecular responses,11, 12 current data do not support any clear difference between TKIs regarding the maintenance of TFR after TKI cessation.13 Recent TKI discontinuation trial data have influenced updates of clinical management guidelines. A minimum of five years of TKI therapy and a stable MR4.0 for ≥2 years before attempting TKI discontinuation is now recommended by the European Society for Medical Oncology (ESMO) and European LeukemiaNet (ELN).14-16 Similar advice regarding stopping TKI outside studies has been issued by the National Comprehensive Cancer Network (NCCN) and the Swedish CML group.17, 18 To date, there are limited data on the outcome and prevalence of TKI discontinuation outside clinical trials. Spanish and Italian groups have recently reported on TKI discontinuation in clinical routine.19, 20 Although these studies provide important information, population-based data could shed more light on the prevalence and real-world outcome after cessation of TKI therapy.
The aim of this study was to use the unique population-based Swedish CML registry to retrospectively identify and characterise patients discontinuing TKI treatment. Further aims were to describe the proportion of patients discontinuing due to DMR with the aim of TFR, the incidence of discontinuation outside of clinical trials and the proportion still treatment-free at time of last follow-up.
Methods
Patients
This is a retrospective cohort study of patients diagnosed with CML in Sweden using the Swedish CML registry. More than 95% of all patients diagnosed with CML in Sweden are reported to the registry.21, 22 The study was approved by the Swedish Ethical review Authority. All patients with a reported diagnosis of CML in chronic phase between 1 January 2007 and 31 December 2012 were identified. Utilising the web-based CML registry, an extra module was created for patients with questions related to TKI discontinuation. Data regarding patients with TKI treatment interruption of ≥1 month were gathered as follows: last TKI prior to discontinuation, reason for TKI discontinuation, date of discontinuation, BCR-ABL1 IS (International Scale) percentage prior to discontinuation, if patient had re-initiated TKI treatment, date and reason of re-initiation and if the patient had discontinued as part of a clinical trial or not. To allow for an adequate TKI treatment time of at least five years, patients diagnosed between 1 January 2007 and 31 December 2012 and with a TKI discontinuation due to DMR (i.e. MR4.0 or better, as reported by treating physician) with TFR as an aim, were selected for further analysis. Date of last follow-up was between 6 March 2018 and 22 November 2019 and median follow-up time was 8·9 years from diagnosis (range 5·6–12·6). Baseline data regarding patient characteristics were collected from the CML registry. The Swedish Prescribed Drug Registry23 was used to identify last prescribed TKI in cases where last TKI was not reported.
Measurements of qRT-PCR of BCR-ABL1
For patients in clinical routine and in the EURO-SKI study qRT-PCR of BCR-ABL1 was measured in laboratories in the University Hospitals in Sweden. These are all certified to express the values on the International Scale. Patients in the ENEST-Freedom and ENEST-Path trials were in this respect evaluated per respective clinical trial protocol.
Statistical methods
Continuous variables are expressed with median and ranges as a measure of variability. Net probability of TKI re-initiation was assessed using the Kaplan–Meier method. The null hypothesis of no difference was tested using a log rank test, and P < 0·05 was considered statistically significant. Patient death was considered as a censoring event. The Mann–Whitney U test was used when comparing time from diagnosis to TKI interruption between patients outside and inside clinical trials. Univariate and multivariate Cox proportional hazard models were used to calculate hazard ratios (HR) with 95% confidence intervals (CI) for the outcome TKI re-initiation, and P values were calculated using the likelihood ratio test. Factors included in the models were age, sex, Sokal risk score, imatinib or second-generation TKI as first-line treatment and at discontinuation, and time in MR4.0 or better prior to discontinuation. A minimal model was chosen using the likelihood ratio test.
Results
Patient selection
There were 584 patients with a reported diagnosis of CML in CP in the Swedish CML registry between January 2007 and December 2012. Information regarding TKI discontinuation was available for 548 (93·8%) patients. A TKI discontinuation of longer than one month was reported for 235 patients. The basis for discontinuation as reported by treating physicians was DMR in 128 (54·5%) (in six of these, pregnancy was also reported), adverse events in 44 (18·7%), allogeneic stem cell transplantation in 32 (13·6%), patient wish in five (2·1%), poor compliance in three (1·3%), pregnancy as sole cause in one (0·4%) and other reasons in 22 (9·4%) patients, respectively. Patients with a reported TKI discontinuation based on a reported DMR were selected for further analysis in this report. Patient characteristics for patients stopping due to DMR are shown in Table I.
Patients discontinuing in DMR
Median time from diagnosis to TKI discontinuation for patients stopping in DMR was 5·3 years (range 1·7–12·3). A majority of the patients (61·7%) did not show any detectable BCR-ABL1 transcripts at the time of discontinuation, while 30·5%, 5·5% and 1·6% of the patients displayed detectable BCR-ABL1 transcript values corresponding to MR4·5, MR4 and MMR, respectively (Table I). Median time from TKI discontinuation to last follow-up was 2·8 years (range 0·1–8·1). By last follow-up, 49·2% (n = 63) had re-initiated TKI treatment, and median time from discontinuation to re-initiation was 4·8 months (range 1·2–44·4). Reason for re-initiation was due to loss of MMR in 58, after completed successful pregnancy in three and due to patient wish and/or physician choice in two patients.
This means that a total of 11·1% (n = 65) of patients diagnosed with CML during 2007–2012 remain treatment-free after a median follow-up of 8·9 years (range 5·6–12·6) from diagnosis.
Discontinuation inside or outside clinical trials
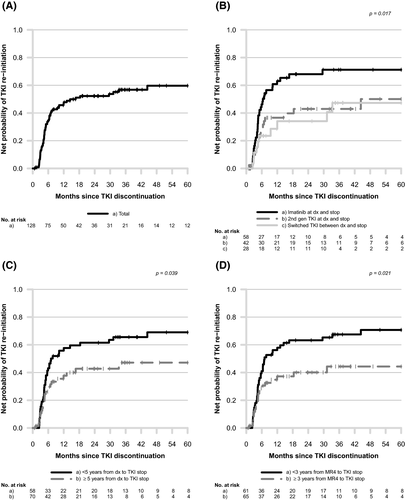
Among the 128 patients discontinuing TKI in DMR, 38 (29·7%) did so as part of a clinical trial and 90 (70·3%) outside a clinical trial. Discontinuation trials performed in Sweden during this time period were EURO-SKI, ENEST-Freedom and ENEST-Path. In the whole DMR cohort the net probability of TKI re-initiation at 12 months was 47·8%. The probability of TKI re-initiation was 41·4% and 73·7% at 24 months and 52·5% and 76·6% at 48 months for patients outside and inside clinical trials, respectively. In all patients discontinuing outside clinical trials, 62·2% (n = 56) were still treatment-free after a median follow-up time of 1·6 years (range 0·1–8·1). Patients discontinuing outside a clinical trial had a longer median time from diagnosis to TKI discontinuation (6·2 years, range: 1·7–12·3) compared with patients in clinical trials (4·2 years, range: 3·1–7·0) (P < 0·001).
Discontinuation in patients on imatinib and second-generation TKI
In 58 (45·4%) patients treatment was imatinib and in 42 (32·8%) patients treatment was second-generation TKI both as first line and at discontinuation, respectively. The remaining 28 (21·9%) patients switched between imatinib and second-generation TKI during their course of therapy. Of these, 21 switched from imatinib to second-generation TKI, and the remaining seven switched from second-generation TKI to imatinib. As shown in Fig 1B, patients treated with second-generation TKI as first-line treatment and at discontinuation had a lower net probability of TKI re-initiation (P = 0·017) compared with patients treated with imatinib. This is despite having a shorter time from diagnosis to TKI discontinuation (4·7 years, range: 2·0–9·3 vs 5·7 years, range: 2·3–12·3) and a larger proportion of patients with Sokal high risk score (40·5% vs 25·9%) as compared with patients treated with imatinib. The group that switched TKI did not differ from the group treated only with second-generation TKI regarding the probability of TKI re-initiation (Fig 1B). HRs for TKI re-initiation were 0·52 (95% CI 0·30–0·93, P = 0·027) and 0·50 (95% CI 0·28–0·90, P = 0·024) for second-generation TKI as compared with imatinib in the univariate and multivariate analysis, respectively. Table II shows the complete list of factors evaluated for the outcome TKI re-initiation.
Duration of TKI therapy and DMR prior to stop
In the whole DMR population, patients who discontinued ≥5 years from diagnosis had a lower probability of TKI re-initiation compared with those who had shorter duration of therapy (Fig 1C). Likewise, patients in DMR (MR4) for ≥3 years prior to TKI stop had lower probability of TKI re-initiation as compared to patients with a shorter time in DMR (Fig 1D), also illustrated by hazard ratios of 0·55 in the univariate and 0·54 in the multivariate analysis (95% CI 0·33–0·92, P = 0·023 and 95% CI 0·32–0·91, P = 0·021 respectively; Table II).
Discussion
In this population-based data set of CML patients in Sweden diagnosed between 2007 and 2012, a large proportion (21·9%) had attempted TKI discontinuation. At the time of TKI stop 97·7% of patients were in MR4 or better as reported by the treating centre, in line with both inclusion criteria in many clinical trials and established guidelines. Seventy per cent of TKI discontinuations during this time period were attempted outside of clinical trials, despite lack of published recommendations at the time of TKI stop. It is well known that TKI discontinuations were attempted before being recommended in clinical guidelines; however, the number was surprisingly high, illustrating the potential benefits of discontinuing TKI treatment as seen by treating physicians and patients early on.
In our study, of all patients discontinuing in clinical practice, 62·2% were treatment-free at last follow-up (median follow-up time 1·6 years), comparing favourably to the TFR rate of approximately 50% in most clinical trials.24 The rate is also similar to the TFR rate described in Spanish and Italian patients (64% at four years, and 69% at 12 months, respectively).19, 20 In the Spanish study, the median TKI treatment time and duration in DMR prior to discontinuation were far longer (around 10 and 5 years respectively) than thresholds for inclusion in clinical trials, likely explaining the high TFR rate in these reports. Similarly, patients discontinuing in clinical practice in our study had longer treatment duration prior to discontinuation (median 6·2 vs 4·2 years) as compared with patients in clinical trials.
In both the univariate and multivariate analysis, treatment with second-generation TKI was associated with a lower probability of TKI re-initiation despite of shorter median treatment duration prior to discontinuation. These results are in line with results from the Italian study where patients with second-generation TKI had a significantly higher probability of remaining in TFR despite shorter treatment time.19 Interestingly, the group of patients that switched between imatinib and second-generation TKI (i.e. treated with second-generation TKI at any time-point) also had a lower probability of TKI re-initiation compared with the imatinib group and similar to the group treated with second-generation TKI only.
Updated CML guidelines from ELN and ESMO recommend that patients have been treated with TKI for at least five years before a TKI discontinuation attempt and also been in DMR for at least two years.15, 16 These recommendations are based on several clinical studies, the largest being the EURO-SKI.4 In this pan-European stop study, duration of TKI therapy and time in DMR were the two important factors to influence the likelihood of remaining in TFR after stopping TKI. In our report patients treated for ≥5 years had a lower probability of TKI re-initiation. Likewise, duration of ≥3 years in DMR prior to discontinuation was associated with a lower probability of TKI re-initiation in both the univariate and multivariate analysis (HR 0·55, P = 0·023 and HR 0·54, P = 0·021). Continued standardised molecular monitoring and re-initiation of TKI therapy in case of relapse is a prerequisite for a successful and safe discontinuation strategy both in clinical trials and in the routine clinical setting. Differences in molecular monitoring between patients stopping in and outside clinical trials in this study cannot be ruled out, with sparser monitoring possibly leading to delayed reporting of molecular relapse.
Our study represents close to all CML-CP patients in Sweden diagnosed in 2007–2012 and provides comprehensive data on prevalence and outcome of TKI discontinuation in a national population-based setting. In this dataset, 11·1% of CML-CP patients were treatment-free by last follow-up. Most estimations of the proportion of CML-CP patients that may achieve TFR are extrapolations from clinical trials or cohort studies.25, 26 Based on cumulative incidences of DMR between approximately 30% and 65% after five years,11, 12 and a TFR rate of 50%, 15–20% of patients with imatinib and 25–30% of patients treated with second-generation TKI have been suggested as the proportions that might ideally attain a TFR. However, basing the estimation on cumulative incidences of DMR tends to overestimate the proportion in stable DMR. In a recent French study of patients treated with TKI outside of clinical trials, cumulative incidence of MR4.0 was 72%, while sustained MR4.0 was reached by 46% after a median follow-up time of seven years.27 In addition, a prerequisite for reaching this optimal TFR rate is a consensus between individual patients and treating physicians that TFR is the goal of the treatment.
With updated guidelines from ELN,15 as well as on the national level regarding TKI discontinuation and longer treatment time resulting in deeper responses, one may assume that discontinuation attempts in clinical practice will increase even further over time. It remains to be seen to what extent the proportion of CML patients achieving TFR will also increase.
In summary, our results from a population-based study show that TKI discontinuation in CML in clinical practice is common and feasible and as successful as when performed within a clinical trial. Our findings are also in line with previous reports of discontinuation of TKI therapy in clinical routine that patients treated with second-generation TKI achieve a higher TFR rate.19, 27
Acknowledgements
The authors would like to thank all Swedish haematologists and study nurses who have reported patients to the Swedish CML registry. We also appreciate the work of data managers at the respective Regional Cancer Centres. HF performed data gathering and analysis and wrote the manuscript. FS extracted registry data and did statistical analysis. MH and JR planned the study and performed data gathering and analysis. TD, AD, AL, BM, KM-E, KO, UO-S, AS, SS, LW, HW and LS gathered data. All authors critically revised the manuscript and approved of the final version.
Conflicts of interest
UO-S has received honoraria from Ariad. The other authors have no disclosures to make.
| TKI discontinuation as part of clinical study | TKI therapy | Total (n = 128) | ||||
|---|---|---|---|---|---|---|
| Yes (n = 38) | No (n = 90) | Imatinib at dx and stop (n = 58) | 2nd gen TKI at dx and stop n = 42) | Switched TKI between dx and stop (n = 28) | ||
| Time (years) from diagnosis to most recent follow-up regarding TKI discontinuation | ||||||
| Median (range) | 9·7 (6·7–12·3) | 8·3 (5·6–12·6) | 9·6 (5·6–12·5) | 8·3 (5·8–11·6) | 9·0 (6·4–12·6) | 8·9 (5·6–12·6) |
| Age (years) at diagnosis | ||||||
| Median (range) | 58 (17–79) | 58 (22–82) | 61 (17–82) | 54 (22–79) | 62 (27–81) | 58 (17–82) |
| Age (years) at TKI discontinuation | ||||||
| Median (range) | 62 (23–83) | 64 (26–89) | 66 (23–89) | 59 (26–85) | 68 (35–88) | 63 (23–89) |
| Sex (%) | ||||||
| Male | 18 (47·4) | 46 (51·1) | 29 (50·0) | 19 (45·2) | 16 (57·1) | 64 (50·0) |
| Female | 20 (52·6) | 44 (48·9) | 29 (50·0) | 23 (54·8) | 12 (42·9) | 64 (50·0) |
| Sokal score at diagnosis (%) | ||||||
| Low | 6 (15·8) | 23 (25·6) | 12 (20·7) | 9 (21·4) | 8 (28·6) | 29 (22·7) |
| Intermediate | 19 (50·0) | 37 (41·1) | 28 (48·3) | 15 (35·7) | 13 (46·4) | 56 (43·8) |
| High | 13 (34·2) | 25 (27·8) | 15 (25·9) | 17 (40·5) | 6 (21·4) | 38 (29·7) |
| Missing | 0 (0·0) | 5 (5·6) | 3 (5·2) | 1 (2·4) | 1 (3·6) | 5 (3·9) |
| TKI therapy (%) | ||||||
| Imatinib at dx and stop | 20 (52·6) | 38 (42·2) | 58 (100·0) | 0 (0·0) | 0 (0·0) | 58 (45·3) |
| 2nd gen TKI at dx and stop | 12 (31·6) | 30 (33·3) | 0 (0·0) | 42 (100·0) | 0 (0·0) | 42 (32·8) |
| Switched TKI between dx and stop | 6 (15·8) | 22 (24·4) | 0 (0·0) | 0 (0·0) | 28 (100·0) | 28 (21·9) |
| Time (years) from diagnosis to TKI discontinuation | ||||||
| Median (range) | 4·2 (3·1–7·0) | 6·2 (1·7–12·3) | 5·7 (2·3–12·3) | 4·7 (2·0–9·3) | 6·0 (1·7–11·4) | 5·3 (1·7–12·3) |
| BCR-ABL1 in % (IS) right before TKI discontinuation | ||||||
| Undetectable (MR4 or better) | 24 (63·2) | 55 (61·1) | 40 (69·0) | 27 (64·3) | 12 (42·9) | 79 (61·7) |
| ≤0·0032 (detectable BCR-ABL1) | 10 (26·3) | 29 (32·2) | 15 (25·9) | 12 (28·6) | 12 (42·9) | 39 (30·5) |
| >0·0032–≤0·01 (detectable BCR-ABL1) | 4 (10·5) | 3 (3·3) | 3 (5·2) | 1 (2·4) | 3 (10·7) | 7 (5·5) |
| >0·01–≤0·1 (detectable BCR-ABL1) | 0 (0·0) | 2 (2·2) | 0 (0·0) | 2 (4·8) | 0 (0·0) | 2 (1·6) |
| Missing | 0 (0·0) | 1 (1·1) | 0 (0·0) | 0 (0·0) | 1 (3·6) | 1 (0·8) |
| Time (years) from diagnosis to MR4 | ||||||
| Median (range) | 1·6 (0·3–4·1) | 1·6 (0·3–8·2) | 2·0 (0·5–7·1) | 1·5 (0·3–5·6) | 1·6 (0·3–8·2) | 1·6 (0·3–8·2) |
| Missing (%) | 0 (0·0) | 2 (2·2) | 0 (0·0) | 1 (2·4) | 1 (3·6) | 2 (1·6) |
| Time (years) from MR4 to TKI discontinuation | ||||||
| Median (range) | 2·5 (0·5–5·8) | 3·7 (0·3–8·4) | 3·1 (0·3–8·4) | 3·0 (0·6–6·8) | 3·2 (0·9–6·4) | 3·1 (0·3–8·4) |
| Missing (%) | 0 (0·0) | 2 (2·2) | 0 (0·0) | 1 (2·4) | 1 (3·6) | 2 (1·6) |
- dx, diagnosis; IS, international scale; MR, molecular response.
| Univariate | Multivariate | Multivariate, minimal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P value | HR | (95% CI) | P value | HR | (95% CI) | P value | |
| Age (years) at TKI discontinuation | |||||||||
| <60 | 1·00 | (ref.) | 0·658 | 1·00 | (ref.) | 0·820 | |||
| ≥60 | 1·12 | (0·68–1·84) | 0·94 | (0·55–1·61) | |||||
| Sex | |||||||||
| Male | 1·00 | (ref.) | 0·882 | 1·00 | (ref.) | 0·836 | |||
| Female | 0·96 | (0·59–1·58) | 0·95 | (0·56–1·59) | |||||
| Sokal score at diagnosis | |||||||||
| Low | 1·00 | (ref.) | 0·559 | 1·00 | (ref.) | 0·542 | |||
| Intermediate | 1·34 | (0·66–2·70) | 1·20 | (0·57–2·54) | |||||
| High | 1·67 | (0·81–3·44) | 1·67 | (0·77–3·61) | |||||
| Missing | 1·55 | (0·43–5·56) | 1·61 | (0·43–5·98) | |||||
| TKI therapy | |||||||||
| Imatinib at dx and stop | 1·00 | (ref.) | 0·027 | 1·00 | (ref.) | 0·024 | 1·00 | (ref.) | 0·024 |
| 2nd gen TKI at dx and stop | 0·52 | (0·30–0·93) | 0·50 | (0·28–0·90) | 0·53 | (0·30–0·93) | |||
| Switched TKI between dx and stop | 0·48 | (0·24–0·97) | 0·48 | (0·24–0·98) | 0·46 | (0·23–0·94) | |||
| Time from MR4 to TKI discontinuation | |||||||||
| <3 years | 1·00 | (ref.) | 0·023 | 1·00 | (ref.) | 0·021 | 1·00 | (ref.) | 0·020 |
| ≥3 years | 0·55 | (0·33–0·92) | 0·54 | (0·32–0·91) | 0·54 | (0·32–0·91) | |||
- dx, diagnosis.