Using depth of response to stratify patients to front line Autologous Stem Cell Transplant: results of the phase II PADIMAC Myeloma Trial
†Collaborators: Shirley D’Sa (Mount Vernon Cancer Centre, Herts), Andres Virchis (Royal Free Hospital Foundation NHS Trust, London), Michael Quinn (Belfast Trust), Charles Crawley (Addenbrookes Hospital, Cambridge), Guy Pratt (University Hospitals Birmingham NHS Foundation Trust, Birmingham), Mark Cook (University Hospitals Birmingham NHS Foundation Trust, Birmingham).
Autologous stem cell transplantation (ASCT) remains the standard of care for younger and fitter multiple myeloma (MM) patients; however, highly effective induction combination regimens have questioned whether ASCT can be deferred. Trials randomising patients to ASCT or not have shown benefit for up-front ASCT.1, 2 A response-stratified approach to treatment has not yet been reported, but is already being practised, mainly in the United States.3
The benefit of ASCT is related to an uplift in major response and minimal residual disease (MRD) negativity, associated with improved progression-free survival (PFS) and overall survival (OS).4, 5 Achieving this with induction chemotherapy alone questions the additional benefit of upfront ASCT. This trial was designed to estimate the PFS of patients who, having achieved a major serological response [≥ very good partial response (VGPR)] to induction therapy, were stratified to receive no further treatment. Neither consolidation for those not proceeding to ASCT nor maintenance post-ASCT were incorporated as these treatments were under evaluation at the time of this protocol and our primary goal was to investigate outcomes using this approach to inform future studies.
PADIMAC was a multi-centre phase II trial for newly diagnosed transplant eligible (NDTE) patients with MM (Supplementary Materials Section 1). Patients received 4–6 cycles of PAD induction6 followed by peripheral blood stem cell harvest (PBSCH). Those achieving ≥VGPR post-PBSCH received no further treatment and were observed until disease progression (‘VGPR-W&W’) when ASCT could be offered. Those achieving PR proceeded to high-dose melphalan (as per institutional practice) and ASCT with no further therapy until disease progression (‘PR-ASCT’). MRD was centrally assessed by multi-parametric flow cytometry (MPFC) with a detection threshold of 0·004%7 at baseline, post-PBSCH and 100 days (D100) following PBSCH/ASCT.
The primary endpoint was to estimate 2-year PFS by MRD status for the VGPR-W&W group. Secondary aims were to assess the response and toxicity of induction, MRD status following induction and at D100, PFS for PR-ASCT patients, PFS2, OS and biomarkers of clinical outcomes.
The trial registered 153 patients (March 2011–January 2014, Table SI; baseline characteristics: Table SII; consort diagram: Figure S1). All patients received ≥1 cycle of PAD induction and 139 (90·8%) received 4–6 cycles (adverse events: Tables SIV and SV).
The overall response rate following induction was 82·4% (126/153), with 63 (41·2%) achieving ≥VGPR (Table SIII, adverse events). Of the 126 patients achieving ≥PR and proceeding to PBSCH, 63 achieved ≥VGPR post-PBSCH. Of the remaining 63 patients, 17 did not proceed directly to PBSCH and came off study (Table SVI), 46 harvested (PR = 44, PD = 2), with 36 proceeding to ASCT (Table SVII).
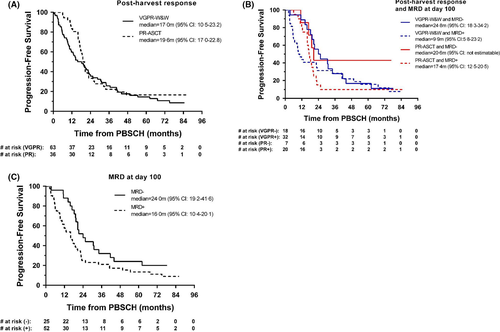
After a median follow-up of 71.4 months from PBSCH, median PFS was 17.0 months (95% CI: 10·5–23·2) for the VGPR-W&W group and 19.6 months (95% CI: 17·0–22·8) for the PR-ASCT group, 2-year PFS was 37·1% (95% CI: 25·3–48·9) and 33·3% (95% CI: 18·8–48·6), respectively (HR = 1·23, 95% CI: 0·79–1·92, P = 0·36; Fig 1A). Within the VGPR-W&W group, by MRD status at D100, median PFS was 24.8 months (95% CI: 18·3–34·2) for MRD-negative (n = 18) and 9.9 months (95% CI: 5·8–23·2) for MRD-positive (n = 32) patients, 2-year PFS was 55·6% (95% CI: 30·5–74·8) and 31·3% (95% CI: 16·4–47·3), respectively (HR = 0·74, 95% CI: 0·40–1·37, P = 0·33; Fig 1B). Consistent with their longer PFS, all 18 MRD-negative patients post-PBSCH remained in ≥VGPR at D100, compared to 8 of 32 MRD-positive patients whose response worsened (3 PR, 5 PD). Investigating PFS by MRD status irrespective of pathway (combining VGPR-W&W and PR-ASCT groups), MRD negative patients at D100 (n = 25) generally had a longer median PFS (24.0 months, 95% CI: 19·2–41·6) than those who were MRD positive (n = 52, 16.0 m, 95% CI: 10·4–20·1) (HR = 0·59, 95% CI: 0·35–1·01, P = 0·05; Fig 1C).
Changes in MRD status from post-PBSCH to D100 by treatment are presented in Table SVIII. Within the VGPR-W&W group, 44 had standard risk and 12 adverse risk genetics. There was no difference in PFS (Supplementary Figure SIII). Although small numbers, 1/8 MRD-negative standard risk and 1/3 MRD-negative adverse risk patients became MRD-positive at D100; 4/24 MRD-positive standard risk patients converted to MRD-negative, whereas the only adverse risk patient remained positive at D100 (Table SIX).
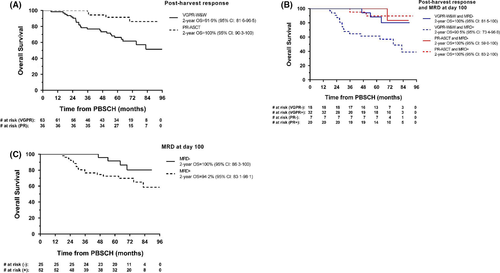
Of the 63 VGPR-W&W patients, 24 died (20: MM, 4: infection), 33 progressed and remain alive and 6 are alive without progression. For the VGPR-W&W group as a whole, 2-year OS was 91·9% (95% CI: 81·6–96·5; Fig 2A); by MRD status at D100, 2-year OS was 100% (95% CI: 81·5–100) for MRD-negative and 90·5% (95% CI: 73·4–96·8) for MRD-positive patients (HR = 0·28, 95% CI: 0·08–0·97, P = 0·04; Fig 2B). Combining the VGPR-W&W and PR-ASCT groups, the 2-year OS rates were 100% (95% CI: 86·3–100, MRD-negative) and 94·2% (95% CI: 83·1–98·1, MRD-positive) (HR = 0·41, 95% CI: 0·14–1·23, P = 0·11); medians have not yet been reached (Fig 2C). PFS2 and additional exploratory analyses, including by cytogenetic risk, are presented in the Supplementary Materials (Figures SII–SV).
PADIMAC was the first prospective trial to investigate whether a subgroup of patients can safely defer ASCT at front-line therapy based on disease response. Our findings suggest that using serological response alone was not adequate to stratify patients, as many in ≥VGPR remain MRD-positive, with short PFS indicating that further cytoreductive therapy is required. Deferred ASCT may, however, be an option for standard risk MRD-negative patients, warranting further investigation. The numbers of adverse risk patients were too small to draw definitive conclusions; however, published data show that even after achieving a deep response, they are less likely to maintain it.8 We recommend MRD-positive patients post-induction should receive ASCT as non-ASCT pathway outcomes were poor. Differences in outcomes for MRD-negative patients in PADIMAC, and those from the IFM and EMN02 studies, are likely attributable to the lack of maintenance therapy and/or the MRD detection threshold and timing of sampling. Consensus guidelines for clinical trials now recommend MRD assessments to at least 10−5.9
Future trials that employ MRD status and cytogenetic risk to stratify patients will help identify patients that can safely be allocated a no-ASCT pathway. Such efforts are especially pertinent today, when the risks associated with the COVID-19 pandemic highlight the need for such a pathway.
Acknowledgements
The authors acknowledge the research staff working across all sites, the patients and their carers. KY and RP are supported by the UCLH/ UCL NIHR Biomedical Research Centre. The trial was coordinated by the CRUK and UCL CTC. Blood Cancer UK conducted an independent review of the study proposal and provided funding. Janssen LLC provided bortezomib free of charge and contributed to trial management costs, but had no involvement in the design, conduct, analysis or interpretation. All authors had full access to the data and the corresponding author was responsible for manuscript submission.
Authorship contributions
KY, RP, JC and RO conceived the study; NC, RP, RD, RO, CR, DDS, EP, TA, NB, PS, LCH, KY analysed the data; RP, JC, GC, MS, SS, MK, JC, MQ, SDS, AV, CC, GP, MC, JL, NR performed the research; RP, NC, KY wrote the manuscript; all authors reviewed and approved the manuscript.
Conflict of interests
RP: Janssen Honoraria and travel support for meetings outside the submitted work; RT: nothing to disclose; NC: grants from Blood Cancer UK, grants and other from Janssen, during the conduct of the study; DDS: nothing to disclose; EP: nothing to disclose; JC: Janssen Honoraria outside the submitted work; TA: grants from Blood Cancer UK, grants and other from Janssen, during the conduct of the study; NB: grants from Blood Cancer UK, grants and other from Janssen, during the conduct of the study; MS: Janssen Honoraria outside the submitted work; SS: nothing to disclose; MK: consultancy and honoraria from Gilead and Alexion; JC: nothing to disclose; MQ: nothing to disclose; TCM: nothing to disclose; SDS: Janssen Cilag Consultancy, Honoraria, education grant and research funding outside the submitted work; AV: nothing to disclose; GC: grants and personal fees from Janssen outside the submitted work; CC: nothing to disclose; GP: grants and personal fees from Janssen outside the submitted work; MC: grants and personal fees from Celgene, personal fees and non-financial support from Takeda, personal fees and non-financial support from Abbvie, personal fees and non-financial support from Amgen, personal fees from Chugai, personal fees from Jazz Pharmaceuticals, personal fees and non-financial support from Janssen UK, outside the submitted work; PS: grants from Blood Cancer UK, grants and other from Janssen, during the conduct of the study; LCH: grants from Blood Cancer UK, grants and other from Janssen, during the conduct of the study; NR: Janssen Consultancy, travel support for meetings and Speakers Bureau outside the submitted work; RO: Janssen Consultancy travel support outside the submitted work; KY: Janssen Honoraria and Research Funding.




