Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study
Summary
Proteasome inhibitors have been associated with thrombotic microangiopathy (TMA) — a group of disorders characterised by occlusive microvascular thrombosis causing microangiopathic haemolytic anaemia, thrombocytopenia and end-organ damage. To date, carfilzomib-associated TMA has predominantly been described in relapsed/refractory myeloma patients. We report eight patients with newly diagnosed myeloma who experienced TMA events while receiving carfilzomib on the phase II CARDAMON trial. The first three occurred during maintenance single-agent carfilzomib, two occurred at induction with carfilzomib given with cyclophosphamide and dexamethasone (KCd) and three occurred during KCd consolidation. At TMA presentation 6/8 were hypertensive; 7/8 had acute kidney injury and in three, renal impairment persisted after resolution of TMA in other respects. The mechanism of carfilzomib-associated TMA remains unclear, though patients with known hypertension seem particularly susceptible. Given the first three cases occurred during maintenance after a longer than five-week treatment break, a protocol amendment was instituted with: aggressive hypertension management, carfilzomib step-up dosing (20 mg/m2 on day 1) at start of maintenance before dose escalation to 56 mg/m2 maximum, and adding 10 mg dexamethasone as premedication to maintenance carfilzomib infusions. No further TMA events occurred during maintenance following this amendment and the TMA incidence reduced from 4·2 to 1·6 per 1 000 patient cycles.
Introduction
Thrombotic microangiopathies (TMAs) are a group of disorders characterised by occlusive microvascular thrombosis, microangiopathic haemolytic anaemia (MAHA), thrombocytopenia and end-organ damage.1, 2 The pathophysiology is related to endothelial injury, platelet activation and subsequent thrombosis within the microvasculature.2 Patients with multiple myeloma (MM) may be at particular risk of developing TMA, triggered by chemotherapy, bone marrow transplantation and disease.3
Bortezomib, carfilzomib and ixazomib are the three licensed inhibitors of the ubiquitin proteasome pathway frequently used in anti-myeloma regimens. Bortezomib-induced TMA has been reported, invariably associated with acute kidney injury (AKI) ranging from a mild creatinine rise to AKI requiring renal support.4-7 Most reported cases were treated with therapeutic plasma exchange (TPE).7 Biopsy-proven renal TMA resolved on stopping bortezomib though recurred in at least one case on drug re-exposure 18 months after the initial episode.6 An additional 21 cases were recently identified, aside from six cases of ixazomib-associated TMA,8 all reporting serious outcomes including hospitalisation (n = 18) and death (n = 2). Renal injury was the commonest serious consequence of TMA (n = 23), with 10 patients needing renal replacement, of which two required long-term dialysis.8
Carfilzomib is an irreversible proteasome inhibitor (PI) which, in combination with either lenalidomide and dexamethasone or dexamethasone alone, is indicated in MM patients who have received at least one prior therapy. Phase I and phase II clinical studies utilising a dose of up to 56 mg/m2 reported good efficacy with an acceptable safety and tolerability profile9 and no instances of TMA. In the phase III, open-label ENDEAVOR trial comparing 56 mg/m2 carfilzomib and dexamethasone to bortezomib and dexamethasone, two TMA cases were reported among 463 subjects treated with carfilzomib.10 Subsequently, there were several published reports of TMA in association with carfilzomib therapy at varying doses (Table I). Most cases were reported in relapsed/refractory MM (RRMM), with only two occurring in newly diagnosed MM (NDMM) patients.11
| Author | Number of patients reported | Chemotherapy regimen(s) | Summary of clinical findings | Treatment | Sequelae |
|---|---|---|---|---|---|
| Qaqish, I. et al. 12 |
2 |
KTd (32 mg/m2) K (23 mg/m2) |
Thrombocytopenia, MAHA and AKI with ADAMTS13 > 50%; TMA on renal biopsy | TPE +/− haemofiltration | Creatinine improved and event resolved with no obvious benefit with TPE |
| Atrash, S. et al. 13 | 1 | K (20 mg/m2) | Thrombocytopenia, MAHA and AKI with ADAMTS13 > 50% | TPE and steroids | Died from TMA 44 days later |
| Hobeika, L. et al. 14 | 1 | KTd (27 mg/m2) | Thrombocytopenia, anaemia and HT. No AKI or MAHA but TMA on renal biopsy | Supportive; no TPE | Improved proteinuria and HT though died from progressive MM |
| Lodhi, A. et al. 15 | 1 | K (regimen unspecified) | Thrombocytopenia, MAHA and AKI with ADAMTS13 > 50%; TMA on renal biopsy | TPE | Normal FBC, LDH and haptoglobin in 3 weeks with improving creatinine |
| Chen, Y. et al. 10 | 4 |
KCd (56 mg/m2) Kd (27 mg/m2) |
Thrombocytopenia, anaemia and AKI with MAHA in 3/4. 2/4 who had ADAMTS13 done were both >50% | Haemodialysis in 2 patients; otherwise supportive care with no TPE | No mortality; complete recovery of platelet count >150 × 109/l with improvement in AKI in all patients |
| Sullivan, M. R. et al. 17 | 1 | Kd (dose unspecified) | Thrombocytopenia, MAHA and AKI with ADAMTS13 > 50%; falls with asterixis and bruising though no HT | TPE and supportive care with IV hydration and antibiotics | Improvement of clinical symptoms and laboratory parameters; discharged after 8 days in hospital |
| Yui, J. C. et al. 18 | 8 |
K (20 mg/m2) KMP (36 mg/m2) Kd (56 mg/m2) K (56 mg/m2)/doxorubicin KPd (27 mg/m2) KM (20 mg/m2) |
ADAMTS13 available in 4/8 patients all >50% | Haemodialysis in 3/8 patients with occasional use of TPE and/or eculizumab | 2/8 patients deceased; 6/8 demonstrated clinical improvement and resolution of TMA after K discontinuation |
| Haddadin, M. et al. 19 | 1 | KPd followed by Kd (dose unspecified) | Thrombocytopenia, MAHA and AKI with ADAMTS13 48% | Haemodialysis and supportive care | Platelets and Hb improved after 7 days though remained dialysis-dependent |
| Monteith, B. et al. 20 | 3 | MCRN003/MYX1 phase 2 clinical trial (KCd with K 20/70 mg2 once weekly) | All had preceding HT with thrombocytopaenia, MAHA and normal ADAMTS13; AKI in 2/3 patients | Patient 1 given high-dose prednisolone; patient 2 given daily TPE and high-dose prednisone; patient 3 given TPE only | All made a complete recovery following cessation of protocol therapy and appropriate treatment |
| Portuguese, A. J. and Lipe, B. 21 | 3 |
KR maintenance in 2/3 patients (Patient 1 K TCD = 464 mg; Patient 2 K TCD = 1826 mg) KCd in 1/3 patient (K TCD = 329 mg) |
Thrombocytopenia, MAHA and AKI in all patients with diarrhoea and oliguria/anuria. ADAMTS13 measured in 2/3 was >90% | Haemodialysis in all patients. 2/3 patients had TPE with eculizumab | No mortality though 2/3 continued to require haemodialysis |
| Bhutani, D. et al. 22 | 1 | KP maintenance (20/56 mg2 weekly) | Thrombocytopenia, MAHA and AKI with ADAMTS13 84% | Haemodialysis and eculizumab | Haemolytic parameters and thrombocytopenia improved after 5 days eculizumab and off dialysis after 16 days |
| Blasco et al. 23 | 4 |
Kd (20 mg/m2 and 56 mg/m2) KRd (27 mg/m2) |
Thrombocytopenia, MAHA and AKI in all patients with ADAMTS13 > 40% | TPE in 3/4 patients and eculizumab in 1/4 with ICU support needed in all patients and 3/4 had haemodialysis | Kidney response seen in all patients, with haematological recovery in 3/4 |
- K, carfilzomib; T, thalidomide; C, cyclophosphamide; d, dexamethasone; R, revlimid/lenalidomide; M, melphalan; P, pomalidomide; TCD, total cumulative dose; HT, hypertension; Hb, haemoglobin; MAHA, microangiopathic haemolytic anaemia; AKI, acute kidney injury; TMA, thrombotic microangiopathy; TPE, therapeutic plasma exchange; IV, intravenous.
We describe the clinical and laboratory features, and outcomes, of the largest patient cohort who developed TMA on carfilzomib as front-line therapy for MM within a prospective clinical trial. We also describe the urgent safety measures put in place during the study, based on early observations of TMA events which occurred at a rate that was higher than expected from previous reports.
Patients and methods
CARDAMON is a phase II, randomised, open-label clinical trial in transplant-eligible NDMM. Patients received bi-weekly carfilzomib (56 mg/m2) in a 28-day cycle (days 1, 2, 8, 9, 15 and 16) with cyclophosphamide and dexamethasone (KCd) as induction therapy, followed by randomisation to standard consolidation with autologous stem cell transplantation (ASCT) or with a further four cycles of KCd. All patients received up to 18 months of maintenance with weekly single-agent carfilzomib (days 1, 8 and 15) at 56 mg/m2 or highest last dose. Patients with uncontrolled hypertension (HT) within 14 days prior to registration were excluded from the trial. Grade ≥ 3 HT while on treatment was managed by stopping carfilzomib until HT was well controlled to grade ≤ 2, and possibly restarting at one dose level reduction as clinically appropriate.
As an adverse event (AE) of special interest, TMA events were reported using a bespoke case report form that captured blood pressure (BP), full blood count (FBC), coagulation screen, biochemistry including lactate dehydrogenase (LDH), and measurement of ADAMTS13 activity. Toxicity was assessed using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. The CARDAMON trial was sponsored by University College London (UCL) and coordinated by the Cancer Research UK and UCL Cancer Trials Centre (ClinicalTrials.gov identifier NCT02315716).
Results
Of 281 patients registered on CARDAMON, eight (2·8%) experienced a TMA (Table II). The median age was 59 years (range 50–71), with a male to female ratio of 6:2. Half had IgG kappa MM, with one IgA kappa and three kappa light chain MM; 2/8 had adverse-risk cytogenetics (Table III). Five patients had a history of HT, including one with concomitant ischaemic heart disease, and two had moderate chronic kidney disease (CKD) with <60 ml/min estimated glomerular filtration rate (eGFR) at baseline. Two of the eight TMA events occurred during the first induction cycle, three during the first cycle of consolidation and three during maintenance — two within the first cycle and one during the fourth. Median time to presentation for all patients was seven months (range 0–15) since starting treatment on CARDAMON. Five of the six TMA cases that occurred beyond induction developed following a period of interruption from chemotherapy, either for stem cell harvest or ASCT. The median time between the last carfilzomib infusion and subsequent carfilzomib treatment at either the start of consolidation or maintenance was 103 days (range 40–208 days). The two patients who experienced TMA at first cycle of maintenance post-ASCT had had the longest carfilzomib-free treatment periods (178 and 208 days).
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Patient demographics | 50 y, male Caucasian | 71 y, male Caucasian |
53 y, female Caucasian |
69 y, male Caucasian |
66 y, male African |
52 y, male Caucasian |
54 y, female Caucasian |
59 y, male Caucasian |
| Cycle |
Maintenance (C4D11) |
Maintenance (C1D1) |
Maintenance (C1D1) |
Induction (C1D11) |
Consolidation (C1D5) |
Consolidation (C1D3) |
Consolidation (C1D9) |
Induction (C1D10) |
| K dose at TMA event | 56 mg/m2 | 56 mg/m2 | 56 mg/m2 | 56 mg/m2 | 56 mg/m2 | 56 mg/m2 | 56 mg/m2 | 56 mg/m2 |
| Symptoms | Nausea and anuria | Nausea, vomiting and fever | Nausea, lethargy and dark urine | Weakness and epistaxis | Anuria and constipation | Nausea, vomiting, diarrhoea intermittent headache & dark urine | Fever and productive cough, followed by acute breathlessness | Nausea and fever |
| History of hypertension | Yes | Yes | No | Yes | Yes | Yes | No | No |
| Hypertension at presentation | Yes | Yes | No | Yes | Yes | Yes | Yes | * |
| Hb (g/l) | 104 (117) | 92 (113) | 101 (120) | 90 (120) | 107 (107) | 98 (131) | 123 (114) | 76 (107) |
| Platelets (×109/l) | 20 (155) | 5 (101) | 13 (197) | 3 (179) | 8 (171) | 88 (195) | 14 (263) | 110 (246) |
| Creatinine (µmol/l) | 746 (72) | 209 (139) | 105 (55) | 203 (68) | 530 (118) | 135 (72) | 444 (73) | 201 (174) |
| LDH (IU/l) | 3000 | 1309 | 2092 | 900 | 3106 | 741 | 1323 | * |
| Blood film | Fragments | Fragments | Fragments | Fragments | Fragments | Fragments | Fragments | No fragments |
| Haptoglobin (g/l) | <0·1 | <0·1 | <0·7 | * | * | <0·1 | 0·2 | * |
| ADAMTS13 | 88% | 148% | 73% | 82% | 68·4% | 74% | 96·6% | * |
| Infection + | None | Fever at presentation but negative cultures | None | None | None | None | Yes – respiratory tract infection | Fever and nausea associated with MAHA |
| Treatment | TPE and haemofiltration | TPE and steroids | TPE | TPE and haemofiltration | TPE and haemofiltration | TPE | TPE and antibiotics | Transfusion and antibiotics |
| Sequelae | CKI for 4 months with progressive improvement | Recovered in 1 week | Recovered in 3 weeks | Recovered in 10 days | Discharged from hospital after 6 weeks on dialysis; off dialysis after 6 months with residual renal impairment | Recovered in 4 days and proceeded to ASCT 6 weeks later off CARDAMON | Resolved after 7 days, with improved creatinine close to baseline after 3 months | Complete resolution with normal creatinine and platelet count recovery after 18 days |
- For Hb, platelet count and creatinine, blood test results preceding the TMA event are presented in brackets next to the results at presentation — with n = blood result at presentation (n = blood result prior to TMA event). Patient 8 was given a diagnosis of TMA based on suggestive clinical features, an acute fall in Hb, new thrombocytopenia with a >50% platelet count reduction and deranged liver function tests which developed immediately after day 9 K infusion.
- TMA, thrombotic microangiopathy; K, carfilzomib; C, cycle; HT, hypertension; Hb, haemoglobin; TPE, therapeutic plasma exchange; CKD, chronic kidney disease.
- *Not available.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Patient demographics | 50 y, Caucasian male | 71 y, Caucasian male | 53 y, Caucasian female | 69 y, Caucasian male | 66 y, African male | 52 y, Caucasian male | 54 y, Caucasian female | 59 y, Caucasian male |
| Haemoglobin at presentation (g/l) | 132 | 113 | 101 | 125 | 120 | 113 | 113 | 112 |
| Creatinine at presentation (µmol/l) | 71 | 109 | 58 | 70 | 121 | 95 | 66 | 133 |
| Disease isotype | Kappa LC | IgG kappa | IgG kappa | IgG kappa | IgG kappa | IgA kappa | Kappa LC | Kappa LC |
| ISS stage | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 |
| Cytogenetic risk by FISH | Standard risk – del(13q) | Standard risk – del(13q) and hyperdiploidy | Standard risk – t(11;14) & +11q | Standard risk – normal FISH | High risk – +1q & del(16q) | Standard risk – TP53 gain and trisomy 17 | Standard risk – IgH loss and 5p gain | High risk – del(17p) [90%], +1q & t(11;14) |
| Randomisation arm | Consolidation | ASCT | ASCT | None | Consolidation | Consolidation | Consolidation | None |
| Best treatment response | VGPR | VGPR | VGPR | N/A | PR | sCR | sCR | N/A |
| Time from treatment start date to TMA onset (months) | 15 | 11 | 10 | 0 | 5 | 6 | 7 | 0 |
| Time from last K infusion to TMA onset (days) | 5 | 1 | 3 | 2 | 1 | 6 | 6 | 1 |
| ≥5-week treatment break preceding TMA | No | Yes – 178 days | Yes – 208 days | No | Yes – 64 days | Yes – 40 days | Yes – 103 days | No |
- Baseline myeloma characteristics in our small patient group are reflective of the general myeloma population. After looking at age, ethnicity, sex, ISS, disease isotype, cytogenetic risk and haemoglobin/creatinine at presentation, no evidence of an association was found between baseline myeloma characteristics and the risk of experiencing K-induced TMA in this small cohort.
- TMA, thrombotic microangiopathy; ISS, International Staging System; LC, light chain; FISH, fluorescence in-situ hybridisation; ASCT, autologous stem cell transplantation; PR, partial response; VGPR, very good partial response; sCR, stringent complete response; N/A, not applicable; K, carfilzomib.
Presenting symptoms included nausea, lethargy, dark urine, anuria, altered bowel habit and headache. Six were hypertensive at presentation — five grade 3 and one grade 2 in severity. Only one patient presenting with a BP of 166/106 mm Hg had no previous HT history (patient 7). The other five were known to have a history of HT; 4/5 had suboptimal BP control while on treatment (three with grade 2 and one with grade 1 HT) though never severe enough to stop or dose-reduce carfilzomib, and 1/5 had well-controlled HT before presenting with a BP of 170/100 mm Hg (patient 1).
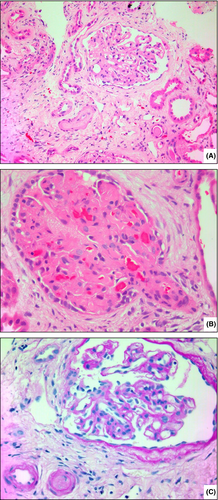
Three were febrile at presentation, one of whom had a confirmed pneumonia. Seven patients presented with AKI: for with stage III, one stage II and two stage I. Renal biopsy performed in patient 5 showed glomerular ischaemic tufts with adjacent vasculopathic arterioles consistent with TMA changes (Fig 1). Seven out of eight had acute thrombocytopenia (median platelets 13 × 109/l, range 3–110), new biochemical evidence of haemolysis with a raised LDH (median 1 323 U/l, range 741–3 106), raised bilirubin (median 17 µmol/l, range 8–130), and blood film morphology consistent with MAHA. ADAMTS13 was assessed in 7/8 cases and all had >50% activity.
Only patient 8 did not have ADAMTS13 measured or MAHA reported on blood film. Bilirubin was normal (8 µmol/l) though LDH and haptoglobin were not assessed for biochemical evidence of haemolysis. Despite this, a decision was made to treat this case as possible TMA based on suggestive clinical features in the absence of proven infection, an acute haemoglobin drop, new thrombocytopenia with a >50% platelet count reduction and deranged liver function tests which developed immediately after day 9 carfilzomib at first cycle induction. The raised alanine amino-transferase (ALT) of 464 IU/l (normal range 10–50 IU/l) with a marginal alkaline phosphatase (ALP) rise of 169 IU/l (normal range 40–129 IU/l) suggests predominant hepatocellular injury. Carfilzomib has been reported to cause increased serum transaminases which resolves on stopping the drug.12 However, liver dysfunction could also have resulted from microvascular endothelial activation and microthrombi causing hepatic injury in the context of TMA.
Three patients had complement levels measured; all had a normal C3 (range 0·9–1.8 g/l) and one patient had a low C4 at 0·03 g/l (range 0·1–0.4 g/l). No genetic mutation studies were done for complement-mediated TMA.
Carfilzomib therapy was withheld in all patients, who were subsequently taken off trial and re-treatment with carfilzomib was not attempted. TPE was initiated in 7/8 patients; one also had corticosteroids. There was no mortality related to the TMA and median time to event resolution/clinical stabilisation was 20 days (range 4–180). The average time for platelet count recovery to levels >150 × 109/l was seven days (range 2–18 days), with longer time for BP to return to baseline (median 12 days, range 6–17).
Renal impairment was the most severe complication, with seven patients presenting with AKI and 3/7 requiring haemofiltration. Four patients had a creatinine rise consistent with a stage III AKI warning at TMA presentation, with only one returning to baseline after 12 days. The other three had a protracted improvement in renal function which stabilised after 90, 120 and 180 days, though none returned to baseline and all developed stage ≥3 CKD. In the 3/7 patients presenting with stage I/II AKI, creatinine levels improved to an eGFR of >60 ml/min/1·73 m2 within a median time of 17 days (range 2–21).
Having observed HT as a common feature in our first four cases, we instituted a protocol amendment with guidance on vigilant BP monitoring, aggressive HT management and appropriate carfilzomib dose reductions should HT remain uncontrolled despite pharmacological measures. We also noted that three of the first four cases occurred during maintenance, where carfilzomib therapy was resumed as single agent after a break of several weeks. No formal assessment was made of the frequency of TMA in patients with pre-existing HT or who had breaks in treatment of more than five weeks. However, these associations were felt to be clinically relevant in the context of a clinical trial utilising 56 mg/m2 bi-weekly carfilzomib. This prompted the guidance issued and further protocol amendments introducing carfilzomib step-up dosing (20 mg/m2 on day 1) at start of maintenance, before escalating to 56 mg/m2 or last tolerated dose on day 8. The original protocol allowed for non-mandatory, low-dose (4 mg) dexamethasone to be given with single-agent carfilzomib maintenance. This was amended to mandate a higher dexamethasone dose (10 mg) on the day of, with a further dose on the day after, carfilzomib infusion. Before the protocol amendment, we observed three TMA events in 714 cycles of carfilzomib treatment (rate of 4·2 per 1 000 cycles). No further TMA events occurred during maintenance following these amendments and the TMA incidence across the entire trial reduced from 4·2 to 1·6 per 1 000 patient cycles (five events in 3 193 cycles). All patients enrolled on trial completed induction and consolidation, with 46 on maintenance at the time of manuscript preparation.
Our findings prompted the instigation of a new urgent safety measure for patients who had their treatment interrupted for four weeks or longer due to the COVID-19 pandemic. Sites were mandated to re-start carfilzomib maintenance at 20 mg/m2 on day 1 before escalating to the patient’s last tolerated dose.
Discussion
Proteasome inhibitor (PI)-associated TMA is a recognised complication of MM therapy, but aetiology and risk factors remain ill-defined. We report eight cases of TMA in NDMM patients who received carfilzomib on the CARDAMON study, seven of whom presented with acute thrombocytopenia, new biochemical evidence of haemolysis, blood film morphology consistent with MAHA and normal ADAMTS13 with >50% activity. Most patients had HT preceding the TMA diagnosis and in seven it occurred either on starting carfilzomib or resuming carfilzomib after a treatment break. Despite stopping therapy and normalisation of haematology parameters, three patients developed CKD. As these occurred in the context of a clinical study, observed associations with poorly controlled HT and prolonged treatment interruption led to rapid institution of protocol amendments to mitigate risk and reduce the incidence of this serious adverse drug reaction.
Drug induced (DI)-TMA secondary to carfilzomib is a recently recognised complication, with 30 cases identified in the literature,11, 13-22, 24 six published as stand-alone case reports14-16, 18, 20, 23 and the rest described within six case series11, 13, 19, 21, 22, 24 (Table I). Most patients (28/30) were treated for RRMM having previously received 1–5 lines of therapy. Only two cases occurred in NDMM patients; they were treated with front-line carfilzomib-based induction within an investigator-initiated study for high-risk MM11 with 10 patients enrolled at the time of reporting. Interestingly, both received carfilzomib at a dose of 20/56 mg/m2 with cyclophosphamide and dexamethasone, presenting early during induction (at C2D2 and C2D8) with MAHA, thrombocytopenia and AKI. One required temporary haemodialysis though both fully recovered, with normal haematological parameters within a week, and renal recovery within a month from stopping carfilzomib. Both were switched to bortezomib-based treatment. In all other reported cases (28/30), carfilzomib doses varied from 20 mg/m2 bi-weekly up to weekly 70 mg/m2, given as a single agent in 4/28 and in conjunction with dexamethasone alone in 6/28 cases. In 18/28, carfilzomib was given as part of various multi-drug combinations including immunomodulatory drugs and alkylating agents (Table I).
We were initially surprised to observe three cases occurring during maintenance, in patients who had previously tolerated treatment well during induction and consolidation. In the literature, the time interval between the first carfilzomib dose and TMA diagnosis varies widely, with the earliest presentation occurring within 24 h of the first single dose of carfilzomib at 20 mg/m214 and the latest recorded at 24 months.20 Similarly, in our patient group, two DI-TMA occurred within 14 days of drug initiation and six occurred afterwards with a median time of 8·5 months (7–15 months). A new observation we make in our series is that 5/6 (83·3%) of carfilzomib-induced TMA which did not occur at the first cycle of induction had a treatment-free period of more than five weeks. This possible increased risk associated with resuming carfilzomib after a treatment break has led to us adopting a vigilant approach to reintroducing the drug after prolonged treatment pauses.
Presenting clinical features and laboratory values in our case series, characterised by a predominance of AKI and persistent renal complications, correlate well with the published literature. Most patients (73·3%) in previously reported cases recovered without sequelae, with platelet counts normalising within days to weeks followed by more gradual improvements in kidney function. Any renal replacement required was often temporary, though three patients were reported to having remained dialysis-dependent.20, 22 There were three deaths out of the 30 cases in the literature, two within 30 days of diagnosis19 and one at 44 days after presentation despite TPE and steroids.14 We had no deaths in our series.
The mechanisms by which carfilzomib-induced TMA occurs have not yet been identified, which adds to the diagnostic challenges and difficulties as illustrated in patient 8. Both immune-mediated and dose-dependent toxicity have been suggested as possible pathological mechanisms of endothelial injury. Also, presentations of DI-TMA with a clinical picture similar to haemolytic uremic syndrome (HUS) are being increasingly described in association with the use of carfilzomib. The effect on complement function is poorly understood, though may involve proteasome-mediated downregulation of alternative pathway inhibitory genes and reduced complement factor H expression,22 with diminished alternative complement pathway inhibition and consequent dysfunction. Patients presenting with AKI, thrombocytopenia and MAHA,22 usually after having previously tolerated at least one treatment cycle,3 were described in the literature, with observations closely resembling those in our cohort. Since atypical HUS (aHUS) is characterised by the same triad of intravascular haemolysis, thrombocytopenia and AKI, this has led to several published reports of carfilzomib-induced aHUS.22-24 Complement levels, including C3 and C4, were generally normal. However, some patients were found to be heterozygous for CFHR3–CFHR1 deletions on genetic testing21 and one had an elevated Bb fragment level with elevation in the soluble membrane attack complex (C5b-9) on a TMA functional panel, reflecting alternate pathway complement activation.23 These patients were treated with eculizumab in an attempt to mitigate disease progression and prevent CKD by blocking the terminal complement pathway. However, evidence of therapeutic benefit shown by normalisation of haematological parameters, improvement in renal function and reduced hospital stays remains limited to anecdotal reports.24
PI-induced vascular endothelial growth factor (VEGF) inhibition16 was also frequently suggested, whereby PIs downregulate key angiogenic factors including VEGF, either via p53 accumulation (VEGF mRNA expression depends on cellular levels of p53),18 or through the inactivation of NF-kB.16 This is consistent with carfilzomib’s well-known cardiorenal effects causing HT, reversible rise in creatinine and common acute rise in N-terminal pro-brain natriuretic peptide (NT-proBNP) in the absence of structural cardiomyopathy.25 These effects may be time- and dose-dependent, as suggested by the increased cardiovascular toxicity in a phase I/II study of weekly carfilzomib at 70 mg/m2 with cyclophosphamide and dexamethasone in transplant-ineligible NDMM26 compared with a previous KCd trial with bi-weekly carfilzomib at 36 mg/m2.27
Bortezomib can cause TMA with comparable clinical features4-7 and a similar AKI predominance explained by the apparent propensity of the glomerular circulation to endothelial damage and occlusion.28 Its pathophysiology is equally elusive, with various mechanisms postulated including complement overactivation causing bortezomib-induced HUS or von Willebrand factor dysfunction causing bortezomib-induced thrombotic thrombocytopenic purpura (TTP).3 However, bortezomib has been shown in vitro to induce a dose-dependent inhibition of endothelial cell proliferation.29 Thus, altered angiogenesis and endothelial damage from suppressed VEGF production and secretion remains a plausible mechanism behind PI-induced TMA.
Vigilant BP monitoring and aggressive HT management was introduced early in the CARDAMON protocol, with appropriate carfilzomib dose reductions for persistent or poorly controlled HT. Having observed that three of the initial four TMA cases occurred during maintenance, carfilzomib step-up dosing was introduced for the first maintenance cycle, with 20 mg/m2 given on day 1 before escalating to the last tolerated dose, and additional dexamethasone introduced during maintenance. These protocol changes were put in as urgent safety measures and, being part of a clinical trial, were relatively easy to implement uniformly in participating treatment centres. A second protocol amendment was introduced during the COVID-19 pandemic, mandating carfilzomib step-up dosing following treatment breaks of at least four weeks during maintenance.
When carfilzomib-induced TMA occurred, immediate discontinuation of the offending drug was the key therapeutic step. This correlates well with other reported cases, whereby the cornerstone of clinical management was stopping carfilzomib together with supportive care, which often involved renal replacement therapy. TPE was employed in eight case series,13, 14, 16, 18, 19, 21, 22, 24 at times with high-dose prednisolone (Table I) and this was, similarly, the mainstay therapeutic strategy in our patient population where empirical TPE was given in 7/8. As with other types of secondary TMA, the role of TPE in the treatment of DI-TMA with ADAMTS13 activity levels >50% is unclear, with no strong evidence of faster resolution or improved treatment outcomes.11, 20 However, we noted a prompt improvement in the haematology parameters and ultimately renal function. Eculizumab is increasingly being used, especially in DI-TMA with clinical presentations similar to HUS.19, 22-24 None of our patients were given eculizumab as it was not available, but this would be the optimal therapy to investigate given the clinical picture. In all, 62·5% of our patients recovered completely without sequelae, though all our affected patients were taken off study and none were re-challenged with carfilzomib.
Conclusion
We report the largest case series of carfilzomib-induced TMA in the NDMM setting, including onset during maintenance even if carfilzomib was previously well tolerated during induction and consolidation. There was no mortality in our case series, though renal co-morbidity was not uncommon, and a history of HT could be a risk factor. We identified initiation and re-initiation after treatment breaks as a potential risk factor for carfilzomib-induced TMA. Supportive care and avoidance of the triggering drug are the only known beneficial management approaches for DI-TMA. However, preventative strategies implemented during the CARDAMON study, including vigilant BP monitoring, dose reduction in uncontrolled HT and step-up dosing with steroid pre-medication at first cycle maintenance following an interruption of four weeks or more in treatment, reduced the incidence from 4·2 to 1·6 per 1 000 patient cycles, with no further cases occurring during carfilzomib maintenance.
Acknowledgements
CARDAMON is coordinated by the CRUK & UCL CTC, funded by Amgen Ltd and endorsed by Cancer Research UK (C9203/A17750). The authors thank sites participating in CARDAMON, local investigators and research teams for their ongoing participation in the study, together with patients and their families.
KY and RP acknowledge support and funding from the UCL/UCLH National Institute for Health Research and Social Care (NIHR) Biomedical Research.




