Infiltration of CD163-, PD-L1- and FoxP3-positive cells adversely affects outcome in patients with mantle cell lymphoma independent of established risk factors
Summary
We characterised patients with mantle cell lymphoma (MCL) with poor prognosis based on differences in immune infiltration. Different expressions of the tumour cell markers Cyclin D1 and sex-determining region Y-box transcription factor 11 (SOX11), and the immune markers cluster of differentiation 3 (CD3), CD4, CD8, CD25, forkhead box protein P3 (FoxP3), T-box transcription factor TBX21 (T-bet), programmed cell death protein 1 (PD-1), programmed-death ligand 1 (PD-L1) and CD163 were investigated for all-cause mortality in 282 patients with MCL and time-to-progression (TTP) in 106 clinical trial patients. With increasing age, a significantly lower infiltration of CD3+ T lymphocytes was seen. T-cell infiltration was independent of cellular tumour antigen p53 (p53) expression, Ki-67, morphology and frequency of tumour cells. The all-cause mortality was higher in patients with PD-L1-expression above cut-off [hazard ratio (HR) 1·97, 95% confidence interval (CI) 1·18–3·25, adjusted for sex and MCL International Prognostic Index (MIPI)] and a higher frequency of CD163+ cells (continuously, HR 1·51, 95% CI 1·03–2·23, adjusting for age, sex, morphology, Ki-67 and p53). In patients treated within the Nordic Lymphoma Group MCL2/3 trials, TTP was shorter in patients with a higher frequency of FoxP3+ cells (HR 3·22, 95% CI 1·40–7·43) and CD163+ cells (HR 6·09, 95% CI 1·84–20·21), independent of sex and MIPI. When combined a higher frequency of CD163+ macrophages and PD-L1+ cells or high CD163+ macrophages and FoxP3+ regulatory T cells indicated worse outcome independent of established risk factors. The T-cell infiltrate was in turn independent of molecular characteristics of the malignant cells and decreased with age.
Introduction
Mantle cell lymphoma (MCL) is one of the most challenging lymphoma subtypes to treat. Although novel treatment options are available and the prognosis is continuously improved,1, 2 the risk of relapse is high, and several high-risk groups have been identified. Age is the strongest negative prognostic factor3, 4 along with blastoid/pleomorphic histology,5-7 high proliferation6 and cellular tumour antigen p53 (p53) overexpression or TP53 mutations.8-11 High-risk patients are also identified by the MCL International Prognostic Index (MIPI).12 Patients having poor prognostic variants respond poorly to novel targeted approaches13, 14 and it has been suggested that immune oncological strategies, including, for example, chimeric antigen receptor (CAR)-T cell treatment15, 16 could improve their outcome.
The attraction of inflammatory cells to the tumour microenvironment in lymphoma tissue is caused by a complex chemokine network that creates tumour supportive niches.17 Cells in the microenvironment support proliferation of the malignant cells in MCL, reduce apoptosis and increase drug resistance.18, 19 Lymphoma cells also acquire different strategies to evade or adapt to the immune system.20 Changes in the number and properties of T cells have a pronounced effect on the immune response,21 and sub-sets of T cells constitute possible therapeutic targets.
In MCL, infiltration of cluster of differentiation 3 (CD3)+, CD4+ and CD8+ T cells is reported to be higher in indolent than in more aggressive cases,22 but the relation to established risk factors has been poorly explored. Cyclin D1+ or sex determining region Y-box transcription factor (SOX11)+ MCL tumour cells overexpress c-c motif chemokine 4 (CCL4) and CCL5 chemokines23 that potentially attract regulatory T cells (Tregs) to the tumour microenvironment as reviewed in Papain et al.17 But the prognostic implication of Tregs in MCL is not well described. Studies of programmed cell death protein 1 (PD-1) and its ligands programmed-death ligand 1 (PD-L1) and PD-L2 in the tumour and immune compartment of MCL are limited. Some studies show no or very low presence of PD-1 expression,24-27 while others show expression in all evaluated tumours.28
Not only T cells, but also tumour-associated macrophages influence tumour development. The macrophages assist the tumour with angiogenesis, stromal modulation and chemokine signalling.29 In cancer tissue, the most common type of tissue infiltrating macrophages are the M2 type that strongly express CD163 protein on their cell membranes.30 Tissue infiltrating macrophages are predictive of poor overall survival (OS) in other lymphoma subtypes,31-33 but their prognostic impact in MCL is unexplored. However, a recent study suggests that there is a crosstalk between MCL cells and CD163-expressing M2 macrophages driven by macrophage colony-stimulating factor 1 (CSF1),34 and that these cells support MCL proliferation. Further investigations could support the potential of M2 macrophages as a future therapeutic target in MCL.
To our knowledge, no previous study has, in parallel, investigated the prognostic impact of different T lymphocytes, PD-1/PD-L1 expressing cells and macrophages in the microenvironment in MCL tumours. This information is relevant for future design of clinical trials that evaluate drugs targeting proteins in the microenvironment. The aim of our present study was to identify MCL subgroups with specific immune cell signatures. Therefore, we explored how immune markers were distributed between patients stratified by established risk factors and investigated if immune cell infiltration was associated with prognosis.
Patients and methods
Study population and immunohistochemistry
The study comprised 282 patients with a diagnosis of MCL, in two (Southern Sweden and Uppsala) out of six healthcare regions in Sweden. The patients were registered in the population-based Swedish Lymphoma Register (SLR) (n = 176) or enrolled in the Nordic Lymphoma Group (NLG) clinical trials MCL2 and MCL3 (NLG-MCL2/3) (n = 106).35, 36 All cases were secondary evaluated as MCL by an haematopathologist. The SLR is a population-based register including patients with MCL at all ages, diagnosed from 2000 to 2017, covering ~95% of patients in the Swedish Cancer Register. Patients enrolled in the NLG-MCL2/3 clinical trials were recruited at hospitals in Denmark, Finland, Norway and Sweden, and were treated with first-line intensive immunochemotherapy35, 36 followed by high-dose therapy with autologous stem cell support. Inclusion criteria for the NLG-MCL2/3 trials were age 18–65 years at diagnosis, no previous cancer treatment, Stage II–IV and tumour cells with cyclin D1 expression or presence of t(11;14).
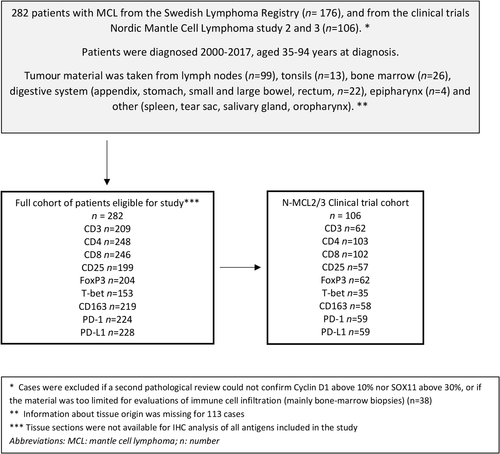
Formalin-fixed paraffin-embedded tumour tissue was used for immunohistochemistry analysis (Fig 1). Details about immunohistochemical staining and evaluation of the tumour cell markers Cyclin D1 and SOX11 and the immune markers CD3, CD4, CD8, CD25, forkhead box protein P3 (FoxP3), T-box transcription factor TBX21 (T-bet), PD-1, PD-L1 and CD163 are outlined in the supplementary text and Table SI.
Information on Ki-67, p53 and morphology was available for most patients, while TP53 mutational status was only available for a sub-set of patients.9, 37 The MIPI was available for the NLG-MCL2/3 cohorts, but only for a limited fraction of the SLR patients, reducing the number of included patients in fully adjusted analyses.
Statistical analysis
For the full cohort of patients with MCL the outcome was all-cause mortality. Time since diagnosis was the underlying time scale. For the homogenously treated patients in the NLG-MCL2/3 cohort, time-to-progression (TTP) was used, with follow-up from date of inclusion or treatment initiation to date of documented death or progression of MCL.
Differences between survival curves were tested using the log-rank test. Uni- and multivariable Cox proportional hazards (PH) models were used to estimate hazard ratios (HRs) for the association between the marker and the all-cause mortality (full cohort) or progression (trial cohort) rates. Three multivariable models were fitted. First, we adjusted for age and sex, then for MIPI and sex, and a fully adjusted model including MIPI, sex, morphology, Ki-67 and p53 expression (for the clinical trial cohort) or age, sex, morphology, Ki-67 and p53 expression (for the full cohort). The TP53 status was not used in the adjusted models due to limited number of patients with sequence information. The PH assumption was formally tested using the Therneau and Grambsch test of the Schoenfeld residuals.38
More detailed information on statistical methods can be found in the supplement. Differences were considered statistically significant for a P < 0·05. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS®) version 25·0 for Windows (IBM Corp., Armonk, NY, USA) and R version 3·6 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical approval
The study was approved by the Ethical Regional Committee in Lund (Dnr 2011/593 for BLISS, Southern Sweden population-based cohort) and Uppsala (Uppsala population-based cohort (Dnr 2014/233) and NLG-MCL2/3 (2009/428).
Results
Patient characteristics
Among the 282 patients, 76% were male and 46% were aged ≥66 years (Table 1). Information about high-risk factors was available for most patients, and 32% had a high MIPI score, 14% had non-classical morphology (pleomorphic or blastoid), 24% had high proliferation (>30% Ki-67 expression) and 11% had high p53 expression (>30% p53-positive cells). Information on TP53 status was available for 55% of the patients, and among those 19% had TP53 mutations. The patients were treated according to different protocols including the NLG-MCL2/3 protocol (57%), rituximab plus bendamustine (R-Bendamustine) (19%) and rituximab-cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate and prednisone (R-CHOP) (12%). The median survival time in the full cohort was 6·9 years and 12·9 years in the MCL2/3 trials (Table 1 and Figure S1).
| Characteristic | Full cohort | NGL-MCL2/3* |
|---|---|---|
| Overall, n (%) | 282 (100) | 106 (100) |
| Sex, n (%) | ||
| Male | 213 (76) | 79 (75) |
| Female | 69 (24) | 27 (25) |
| Age at diagnosis, years, n (%) | ||
| ≤65 | 152 (54) | 106 (100) |
| >65 | 130 (46) | 0 |
| MIPI, n (%) | ||
| Low risk | 79 (40) | 60 (57) |
| Medium risk | 57 (29) | 26 (25) |
| High risk | 63 (32) | 20 (19) |
| Missing | 83 | 0 |
| Morphology, n (%) | ||
| Classic | 228 (86) | 83 (78) |
| Blastoid/pleomorphic | 38 (14) | 23 (22) |
| Missing | 16 | 0 |
| Ki-67, n (%) | ||
| <30% | 210 (76) | 79 (75) |
| >30% | 66 (24) | 27 (25) |
| Missing | 6 | 0 |
| TP53, n (%) | ||
| Wild-type | 108 (81) | 56 (84) |
| Mutated | 25 (19) | 11 (16) |
| Missing | 149 | 39 |
| p53, n (%) | ||
| <30 | 235 (89) | 89 (89) |
| >30 | 29 (11) | 11 (11) |
| Missing | 18 | 6 |
| Treatment***, n (%) | ||
| NLG-MCL2/MCL3 | 135 (57) | 106 (100) |
| CHOP alternating with cytarabine | 12 (5) | 0 |
| CHOP | 28 (12) | 0 |
| Chlorambucil | 6 (3) | 0 |
| R-Bendamustine | 45 (19) | 0 |
| Other treatment** | 9 (4) | 0 |
| Missing | 47 | 0 |
| Overall survival time, years, median | 6·9 | 12·9 |
| Time to progression, years, median | – | 8·7 |
- MIPI, Mantle Cell Lymphoma International Prognostic Index; NLG-MCL2/MCL3 protocol, Nordic lymphoma group – Mantle Cell Lymphoma 2 and 3 protocol containing dose-intensified cyclophosphamide, vincristine, doxorubicin and prednisone (CHOP) in combination with rituximab (R) alternating with rituximab and high-dose cytarabine.
- * NLG-MCL2/3 patients are also included in the full cohort, thus this consists as a subgroup of all.
- ** Other included lenalidomide + bendamustine, radiotherapy, cytarabine, steroids and no treatment.
- *** Most patients were also treated with rituximab [106 (100%) of those in the trial cohort and 207 (73%) of those in the full cohort]. For some patients only data on rituximab+/− was available but not data on given chemotherapy. Due to rounding not all percentages add up to 100.
The composition of the MCL tumour microenvironment
Tumour markers cyclin D1 and SOX11 were highly expressed in most tissue (Fig 2), and their expression correlated with each other (Figure S2). As expected, among the T-cell antigen, the pan T-cell marker CD3 showed the highest median frequency of positive cells (10·3%). Cells expressing immune checkpoint receptors/ligands PD-1 and PD-L1 were both present in the tumour tissue with a median frequency of 10% and 1% respectively. The expression of these markers in relation to previous studies is summarised in Table SII. The frequency of most immune cells was independent of the degree of tumour infiltration, as measured by cyclin D1 expression, in the analysed tissue (Figure S2).
T-cell markers expressions correlated with each other, with CD25 as an exception. One of the strongest correlations was seen between CD4 and FoxP3 (r = 0·6) (Figure S2), suggesting that a large proportion of the CD4+ cells are FoxP3+ Tregs. Also, the Type 1 T-helper cell (TH1) T-cell marker T-bet correlated with FoxP3 (r = 0·5).
CD163+ macrophages were present in very low numbers, with a median expression of 0·06% of the examined cells (Fig 2).
For most patients, biopsies were taken from lymph nodes. However, for some patients, secondary lymphoid tissue or bone marrow was sampled. We found that the distribution between high and low expression of several of the immune markers varied statistically between compartments (Table SIII), but when we plotted the absolute number of cells the distribution was very similar regardless of compartment (Figure S3). Representative staining of the CD4, FoxP3, PD-L1 and CD163 below and above the established cut-offs are shown in (Figure S4).
Association between established risk factors and immune markers
The expression of several immune markers was associated with age, with a richer infiltrate of cells expressing CD3, CD8 and CD25 and a lower infiltrate of PD-1 expressing cells in patients aged ≤65 years (Table II). Besides age, most immune markers did not correlate with the other established prognostic factors such as morphology, Ki-67 or p53 expression. However, PD-L1 and CD163 expressing cells were slightly more common in tumours with non-classical (blastoid or pleomorphic) morphology. T-bet and CD163 were enriched in highly proliferative tumours (Ki-67 >30%) (Table II). We found that most markers, including CD163, were independent of p53 expression and TP53 mutational status. The exception was PD-L1 expression that was different in TP53-mutated compared to wild-type cases (P = 0·035). Tumours harbouring the mutation had more PD-L1 expression, which is well described in other malignancies.39 The relationship between the immune markers PD-L1 and CD163, and high-risk factors is illustrated in Figure S5a and S5b.
| Classic, n (col %) | Non-classic, n (col %) | P | Ki-67 <30%, n (col %) | Ki-67 ≥30%, n (col %) | P | p53 <30%, n (col %) | p53 ≥30%, n (col %) | P | Age ≤65 years, n (col %) | Age >65 years, n (col %) | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Cut-off, % | n (%) | OS, years | 228 | 38 | 210 | 66 | 235 | 29 | 152 | 130 | ||||
| CD3 | ≥10·6 | 103 (50) | 7·5 | 85 (48) | 10 (48) | 1 | 80 (50) | 23 (51) | 1 | 83 (51) | 10 (40) | 0·31 | 63 (65) | 40 (36) | <0·001 |
| <10·6 | 105 (50) | 4·5 | 92 (52) | 11 (52) | 79 (50) | 22 (49) | 80 (49) | 15 (60) | 35 (35) | 70 (64) | |||||
| CD4 | ≥5·5 | 108 (44) | 5·0 | 91 (45) | 15 (43) | 0·85 | 83 (44) | 25 (44) | 0·92 | 92 (44) | 13 (45) | 0·95 | 59 (43) | 49 (45) | 0·78 |
| <5·5 | 140 (56) | 9·2 | 113 (55) | 20 (57) | 105 (56) | 32 (56) | 116 (56) | 16 (55) | 79 (57) | 61 (55) | |||||
| CD8 | ≥2·5 | 200 (82) | 8·1 | 168 (84) | 27 (77) | 0·32 | 150 (81) | 47 (82) | 0·82 | 166 (81) | 24 (83) | 0·82 | 118 (86) | 82 (75) | 0·04 |
| <2·5 | 45 (18) | 4·5 | 32 (16) | 8 (23) | 35 (19) | 10 (18) | 39 (19) | 5 (17) | 19 (14) | 26 (25) | |||||
| CD25 | ≥0·5 | 115 (58) | 6·6 | 104 (61) | 10 (48) | 0·23 | 92 (60) | 23 (53) | 0·43 | 98 (59) | 14 (58) | 0·95 | 61 (66) | 54 (50) | 0·02 |
| <0·5 | 84 (42) | 3·7 | 66 (39) | 11 (52) | 61 (40) | 20 (47) | 68 (41) | 10 (42) | 31 (34) | 53 (50) | |||||
| FoxP3 | ≥5·6 | 22 (11) | 6·3 | 22 (12) | 0 (0) | — | 21 (13) | 2 (4) | 0·10 | 170 (72) | 24 (86) | 0·11 | 12 (13) | 10 (9) | 0·46 |
| <5·6 | 182 (89) | 5·6 | 155 (88) | 21 (100) | 136 (83) | 43 (96) | 67 (28) | 4 (14) | 84 (87) | 98 (91) | |||||
| T-bet | ≥0·12 | 113 (74) | 5·0 | 96 (71) | 14 (93) | 0·07 | 84 (70) | 28 (88) | 0·05 | 91 (72) | 16 (84) | 0·25 | 45 (69) | 68 (77) | 0·26 |
| <0·12 | 40 (26) | 6·7 | 39 (29) | 1 (7) | 36 (39) | 4 (12) | 36 (28) | 3 (16) | 20 (31) | 20 (23) | |||||
| PD-1 | ≥4·2 | 164 (73) | 4·8 | 141 (73) | 16 (70) | 0·66 | 127 (76) | 36 (68) | 0·27 | 137 (73) | 20 (80) | 0·42 | 68 (67) | 96 (79) | 0·04 |
| <4·2 | 60 (27) | 8·3 | 50 (27) | 7 (30) | 41 (24) | 17 (32) | 52 (27) | 5 (20) | 34 (33) | 26 (21) | |||||
| PD-L1 | ≥1·1 | 108 (47) | 3·4 | 88 (45) | 16 (67) | 0·05 | 77 (45) | 31 (60) | 0·06 | 86 (45) | 16 (64) | 0·07 | 46 (44) | 62 (50) | 0·38 |
| <1·1 | 120 (53) | 7·4 | 106 (55) | 8 (33) | 96 (55) | 21 (40) | 106 (55) | 9 (36) | 58 (56) | 62 (50) | |||||
| CD163 | ≥0·6 | 23 (11) | 2·6 | 14 (7) | 7 (29) | <0·001 | 13 (10) | 9 (18) | 0·04 | 15 (8) | 5 (20) | 0·06 | 12 (12) | 11 (9) | 0·51 |
| <0·6 | 196 (89) | 5·6 | 176 (93) | 17 (71) | 153 (90) | 42 (82) | 169 (92) | 20 (80) | 88 (88) | 108 (91) |
- FoxP3, forkhead box P3; OS, overall survival (median); PD-1, programmed cell death protein 1; PD-L1, programmed-death ligand 1; T-bet, T-box expressed in T cells.
- Significant correlations (chi-square test) are highlighted in bold.
Prognostic immune markers in MCL
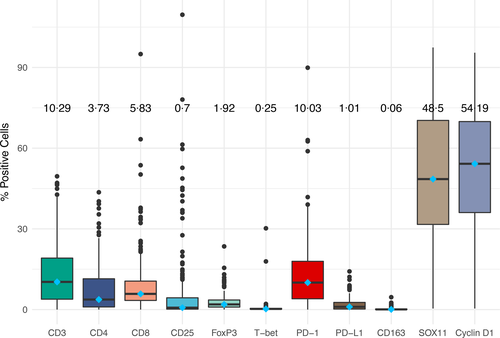
Based on the univariable models, the higher frequency of CD4-, PD-L1- and CD163-positive cells was associated with significantly greater all-cause mortality (Table III). Expression of high CD3 was associated with lower all-cause mortality in univariable, but not multivariable, models. When adjusting for sex and MIPI, higher frequency of CD4 [HR 1·94, 95% confidence interval (CI) 1·15–3·25], PD-L1 (HR 1·97, 95% CI 1·18–3·28) and CD163 (HR 2·5, 95%CI 1·20–5·14) were significantly associated with higher all-cause mortality (Table III). Stratification of patients according to CD163 expression confirmed that patients with expression above the cut-off had a shorter all-cause mortality (Fig 3a). Higher frequencies of CD4 and CD163 remained statistically significant in a sensitivity analysis including only patients treated with R-Bendamustine, the most common treatment outside of the trial cohort (Table SV). CD163 as a continuous variable (HR 1·52, 95%CI 1·03–2·23) was the only marker associated with all-cause mortality when adjusting for age, sex and the tumour biological characteristics morphology, Ki-67 and p53.
| All cohort | Cut-off, % | HR† (95% CI) | P | N | HR‡ (95% CI) | P | N | HR# (95% CI) | P | N |
|---|---|---|---|---|---|---|---|---|---|---|
| CD3 | 10·6 | 0·57 (0·39–0·84) | 0·004 | 208 | 0·68 (0·47–1·01) | 0·06 | 208 | 0·84 (0·47–1·51) | 0·56 | 130 |
| CD4 | 5·5 | 1·55 (1·08-2·21) | 0·02 | 248 | 1·36 (0·95–1·95) | 0·10 | 248 | 1·94 (1·15–3·25) | 0·01 | 171 |
| CD8 | 2·5 | 0·66 (0·42–1·02) | 0·06 | 245 | 0·68 (0·43–1·06) | 0·09 | 245 | 0·94 (0·46–1·93) | 0·87 | 167 |
| CD25 | 0·5 | 0·70 (0·48–1·03) | 0·07 | 199 | 0·74 (0·50–1·08) | 0·12 | 199 | 1·04 (0·57–1·91) | 0·89 | 122 |
| FoxP3 | 5·6 | 0·76 (0·37–1·56) | 0·45 | 204 | 0·65 (0·31–1·35) | 0·25 | 204 | 0·51 (0·18–1·46) | 0·21 | 129 |
| T-bet | 0·12 | 1·51 (0·92–2·5) | 0·10 | 153 | 1·31 (0·78–2·18) | 0·31 | 153 | 1·09 (0·51–2·35) | 0·83 | 87 |
| PD-1 | 4·2 | 1·45 (0·96–2·19) | 0·08 | 224 | 1·24 (0·82–1·89) | 0·31 | 224 | 1·69 (0·94–2·83) | 0·08 | 150 |
| PD-L1 | 1·1 | 1·67 (1·16–2·39) | 0·006 | 228 | 1·46 (1·01–2·10) | 0·04 | 228 | 1·97 (1·18–3·28) | 0·01 | 152 |
| CD163 | 0·6 | 1·91 (1·11–3·3) | 0·02 | 219 | 1·87 (1·07–3·26) | 0·03 | 219 | 2·48 (1·20–5·14) | 0·02 | 145 |
| CD163 cont. | — | 1·51 (1·14–1·99) | 0·004 | 219 | 1·60 (1·20–2·13) | 0·002 | 219 | 1·61 (1·11–2·35) | 0·01 | 145 |
- CI, confidence interval; FoxP3, forkhead box P3; HR, hazard ratio; PD-1, programmed cell death protein 1; PD-L1, programmed-death ligand 1; T-bet, T-box expressed in T cells.
- Results are presented using a cut-off obtained through max-rank statistics. Results are based on the maximal number of cases with complete information and indicated.
- Significant values are highlighted in bold.
- * Estimates for the fully adjusted model (age, sex, morphology, p53 and Ki-67) are given in the text.
- † Estimated from a Cox proportional hazards model (univariable).
- ‡ Estimated from a Cox proportional hazards model adjusted for time since diagnosis (as time scale), age at diagnosis and sex.
- # Estimated from a Cox proportional hazards model adjusted for time since diagnosis (as time scale), sex and Mantle Cell Lymphoma International Prognostic Index.
To assess additive prognostic value of combining markers identified to be independently associated to outcome, patient samples were stratified according to the combined PD-L1 and CD163 expression (Fig 3b). Patients with both markers above cut-off had significantly higher all cause mortality than patients with both markers below cut-off. The same trend of worse prognosis was seen in patients with tumours with expression of both markers above the cut-off compared to patients with tumours with low expression of one of the markers, although not significantly separated.
Immune markers associated with TTP among homogeneously treated MCL patients
The association between each marker and TTP was assessed in patients treated within the NLG-MCL2/3 trials, to investigate how the current ‘gold standard’ therapy for young and fit patients with MCL is affected by immune infiltration. The PH assumption was violated, the HRs were non-proportional for CD3, CD4, CD8, FoxP3 and CD163. This deviation was not considered to change the relevance of the TTP analysis, as this was likely due to the limited number of relapses after 6 years. That is, the HRs presented below are to be interpreted as the average effects across the full follow-up.
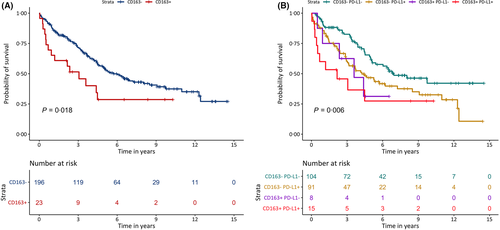
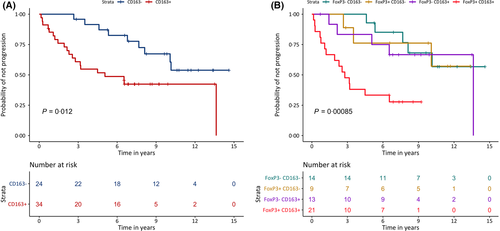
Higher levels of CD3, CD4, CD8, FoxP3 and CD163 were associated with shorter TTP both in univariable models and in age- and sex-adjusted models (Table IV). Additionally, CD163 was associated with TTP when evaluated as a continuous variable (adjusted for sex and MIPI) (HR 6·09, 95% CI 1·84–20·21). Stratification between patients with higher and lower expression of CD163 showed that tumours with increased levels of CD163 progressed within a shorter time than tumours with lower levels of CD163 (Fig 4a). PD-1 was not associated with TTP based on univariable analysis, but when adjusting for MIPI and sex a decreased HR was found (HR 0·42, 95% CI 0·19–0·96).
| Cut-off, % | HR† (95% CI) | P | N | HR‡ (95% CI) | P | N | HR# (95% CI) | P | N | |
|---|---|---|---|---|---|---|---|---|---|---|
| CD3 | 20·1 | 2·50 (1·18–5·28) | 0·02 | 62 | 2·60 (1·21–5·60) | 0·01 | 62 | 1·84 (0·80–4·21) | 0·10 | 62 |
| CD4 | 7·1 | 2·02 (1·14–3·57) | 0·02 | 103 | 2·01 (1·13–3·59) | 0·02 | 103 | 1·60 (0·88–2·89) | 0·20 | 103 |
| CD8 | 16·1 | 2·14 (1·14–3·99) | 0·02 | 102 | 2·21 (1·13–4·30) | 0·02 | 102 | 1·51 (0·78–2·93) | 0·20 | 102 |
| CD25 | 3·9 | 0·70 (0·26–1·86) | 0·47 | 57 | 0·66 (0·24–1·81) | 0·40 | 57 | 0·81 (0·30–2·19) | 0·70 | 57 |
| FoxP3 | 2·0 | 3·03 (1·37–6·7) | 0·006 | 62 | 3·05 (1·37–6·76) | 0·006 | 62 | 3·22 (1·40–7·43) | 0·006 | 62 |
| T-bet | 0·07 | T-bet upper limit is infinite | ||||||||
| PD-1 | 2·5 | 0·46 (0·21–1·02) | 0·06 | 59 | 0·46 (0·21–1·01) | 0·05 | 59 | 0·42 (0·19–0·96) | 0·04 | 59 |
| PD-L1 | 0·4 | 2·34 (0·99–5·53) | 0·05 | 59 | 2·32 (0·98–5·53) | 0·06 | 59 | 2·30 (0·96–5·50) | 0·06 | 59 |
| CD163 | 0·04 | 2·71 (1·2–6·07) | 0·02 | 58 | 2·83 (1·25–6·42) | 0·01 | 58 | 2·21 (0·45–5·12) | 0·06 | 58 |
| CD163 cont. | — | 6·35 (2·33–19·10) | 0·001 | 58 | 12·87 (3·35–50·88) | <0·001 | 58 | 6·09 (1·84–20·21) | 0·03 | 58 |
- CI, confidence interval; FoxP3, forkhead box P3; HR, hazard ratio; PD-1, programmed cell death protein 1; PD-L1, programmed-death ligand 1; T-bet, T-box expressed in T cells.
- Results are presented using a cut off obtained through max-rank statistics. Results are based on the maximal number of cases with complete information and indicated.
- Significant values are highlighted in bold.
- * Estimates for the fully adjusted model [Mantle Cell Lymphoma International Prognostic Index (MIPI), sex, morphology, p53 and Ki-67] are given in the text.
- † Estimated from a Cox proportional hazards model (univariable).
- ‡ Estimated from a Cox proportional hazards model adjusted for time since diagnosis (as time scale), age at diagnosis and sex.
- # Estimated from a Cox proportional hazards model adjusted for time since diagnosis (as time scale) sex and MIPI.
In the fully adjusted model including MIPI, sex, morphology, p53 and Ki-67, PD-L1 stood out as significantly associated with shorter TTP in the clinical trial cohort (HR 2·65, 95% CI 1·05–6·68). The average TTP for the PD-L1 low and high groups was 11·3 and 9·3 years respectively.
Increased frequency of FoxP3+ cells had an impact on TTP with a HR of 3·22 (95% CI 1·40–7·43) when adjusted for sex and MIPI (Table IV), indicating that Tregs are important for treatment outcome when high-dose chemotherapy and rituximab are used.
To assess the combined risk of the two markers with the greatest impact on prognosis, the patient samples were stratified according to the combined FoxP3 and CD163 expression (Fig 4b). An additive effect of the two markers was seen and patients with tumours with higher expression levels for both markers were associated with having a shorter TTP compared to single positive tumors.
Discussion
Immune-mediated control of malignant cells can limit the spread and proliferation of tumours.20 Recent development of immune-oncology strategies that harness the potential of the immune system have revolutionised how cancer is treated.40 Thus, improved understanding of immune infiltration is key to develop and apply novel strategies and to select optimal treatment for specific sub-types of cancer.
The MCL microenvironment has been proposed to use both immune re-education and escape mechanisms,20 and the aim of the present study was to better understand how immune infiltration contributes to prognostication beyond already established risk factors, and suggest which markers have potential as future therapeutic targets.
Even though CD163+ macrophages were present at low frequency, we show for the first time that a continuous increasing of CD163 expression contributed independently to all-cause mortality in a multivariable model. CD163 expression seemed independent of T-cell infiltration, but was significantly associated with blastoid morphology. In other lymphoma entities a higher infiltrate of CD163+ macrophages has also been associated with poor outcome.31, 32 The prognostic implication of CD163 in follicular lymphoma is different depending on investigation before or after introduction of rituximab,41 indicating that response to anti-CD20 treatment may be dependent on the presence of macrophages. In our present study, presence of CD163+ cells indicated both higher all-cause mortality in a cohort treated with a wide range of therapies, and shorter TTP in the well-defined clinical trial cohort, with patients treated with rituximab and intensive chemo-immunotherapy and autologous stem cell transplantation. As CD163+ cells were expressed in low numbers, the associated negative effect on outcome may be a surrogate marker for a disadvantageous microenvironment with reduced tumour cell apoptosis and/or enriched growth factors. Others have described a crosstalk, where MCL cells secrete CSF1 that promote the conversion of macrophages into CD163-expressing M2 macrophages that support proliferation of tumour cells.34 Of interest, this crosstalk could be disrupted using either ibrutinib or CSF1-inhibitors with an additive effect when combined, constituting an interesting treatment strategy for patients with presence of CD163+ cells in their MCL tumour microenvironment.
Expression of the checkpoint protein PD-1 could be detected, but did not show broad independent prognostic impact. However, a higher frequency of PD-L1+ cells in the tumour microenvironment was associated with shorter TTP in fully adjusted analyses in the NLG-MCL2/3 cohort. This indicates that even for treatments not including PD-L1 inhibition, the outcome was affected by the expression of this immune suppressive antigen and independent of most established risk factors, with TP53 status as an exception. Earlier studies indicate that MCL tumour cells and cells in the microenvironment express PD-L1, but results have been inconclusive.25-27 This may be due to a small cohort size, different antibody clones used, or the variation with age and biopsy site that we show. Our present cohort of 282 patients is the largest collection of patients with MCL to date, investigating de novo PD-1/PD-L1 expression in diagnostic biopsies. Cell surface expression of PD-1/PD-L1 seems to be critical for the identification of other non-Hodgkin lymphoma patients eligible for immune checkpoint blockade therapies.42 Based on our present results, we could speculate that a subset of patients with MCL would respond to checkpoint blockade using PD-L1, and efficient companion diagnostic methods are needed to identify those patients. Our combined Kaplan–Meier analysis of patients having CD163 and PD-L1 expression above cut-off indicate that patients with tumours expressing both antigens at higher levels have poor prognosis and they could be considered for more intensive treatments.
Several other markers were associated with poor outcome in univariable analyses of all-cause mortality and TTP, such as CD4. However, in most adjusted models CD4 was not independently significant, indicating that other factors affect both CD4 expression and prognosis. In a previous study, low absolute CD4+ T-cell count in peripheral blood was identified as a negative prognostic factor.43 In addition, Nygren et al.22 showed, using flow cytometry, that a high CD4:CD8 ratio correlated independently with long OS (n = 243) in MCL treated with various regimens. It is difficult to compare these studies with our present results as we have used intact tissue that may contain CD4+ macrophages in addition to T cells, and not blood or cells isolated from tissue that would allow flow cytometry analysis.
In addition to study markers for pan T cells (CD3)-, CD4- and CD8-positive cells, we analysed CD25 and FoxP3 to enable assessment of Tregs. Tregs are in focus for immune-oncology strategies, as they are able to re-educate the microenvironment. Multiple types of Tregs have been described in B-cell malignancies44, 45 Malignant B cells actively recruit FoxP3+CD25+ Tregs and among biopsy specimens of B-cell non-Hodgkin lymphoma, Tregs are enriched (median of 17% in lymphoma biopsies, 12% in inflammatory tonsil, and 6% in tumour-free lymph nodes).46 In the homogenously treated NLG-MCL2/3 cohort, FoxP3+ cells showed strong independent association to poor outcome. This indicates that the prognostic effect of Tregs is dependent on the treatment provided. When combining information about CD163 and FoxP3, a sub-group of patients with increased expression of both markers and with increased risk of progression was identified, indicating that macrophages and T cells additively contribute to a disadvantageous microenvironment.
Many previous studies of immune infiltration have not adjusted for already established risk factors, such as MIPI,47 which is important to understand if markers contribute to improved prognostication beyond what is clinically established. High infiltration of FoxP3+ cells in addition to increased CD163 remained significantly associated with shorter TTP in adjusted analyses with established risk factors, including MIPI, in the trial cohort. Thus, we propose that, in the era of immune-oncology, diagnostic methods where the degree of immune infiltration is assessed will be needed to complement MIPI for accurate prognostication and/or treatment selection.
Our present results also indicate that the microenvironment is very heterogeneous and with large inter-individual variability. The immune variation associated with age has been shown outside of lymphoma studies earlier,48 but the distribution between biopsy sites have not previously been reported in this context. Thus, we identify age as an important confounding factor that needs to be considered in future studies of the MCL microenvironment. The heterogeneity in tumour tissue might also be open for targeted approaches to a specific immune tumour microenvironment, such as for example PD-1/PD-L1. A wide range of novel targeting therapies are being evaluated for B-cell lymphomas, including cellular15 and antibody-based therapies with the aim to direct immune response towards malignant cells.49
The well-known poor prognosis for elderly patients with MCL was confirmed. This is likely explained by the lack of tolerance to effective treatment due to comorbidities,50 but potentially also caused by tumour biological differences between younger and older patients. Our findings of different T-cell subsets showing a lower infiltrate in elderly patients may indicate less ability to mount an effective T-cell response with increasing age. The present study cannot state if any specific immune-related treatment would be best suited for any age groups. However, the change in the tumour microenvironment’s T-cell content with age indicate possibilities for treatments boosting T-cell response, such as CAR-T cell therapies,15 potentially with different approaches in different age groups.
In conclusion, our present study provides a broad characterisation of the tumour microenvironment in MCL that has been lacking. The population-based approach provides a real-life view of different patients with MCL, including young and fit, and elderly and frail. The finding of CD163+ macrophages correlating with poor prognosis was similar for both all-cause mortality and TTP, emphasising the importance of further investigations of how the crosstalk between MCL and M2 macrophages can be addressed. For some of the other immune markers, e.g. FoxP3, the microenvironment impact on prognosis was more pronounced for patients with homogenous treatment.
We propose that CD163, PD-L1 and FoxP3 are of major interest for further evaluation as tools for stratification of patients with MCL beyond established risk scores. Our present results also indicate that future trials testing different immunotherapies in MCL should be stratified by degree of tumour immune infiltration.
Acknowledgements
We would like to acknowledge the technical support of FoU-department at the pathology department, Uppsala University Hospital and Kristina Lövgren, Department of Oncology and May Hassan, Department of Immunotechnology, Lund University.
Conflict of interest
Ingrid Glimelius has received honoraria from Janssen-Cilag (not related to this study). Mats Jerkeman has received research support from Roche, Abbvie, Celgene, Janssen, Gilead. Honoraria from Roche, Kiowa Kirin, Abbvie, Celgene, Janssen, Gilead (not related to this study).
Funding information
The study was supported by grants from Swedish Cancer Society (16/465 and 19/0309, to Sara Ek; 19 0123 Pj 01 H and 19 0109 SCIA, to Ingrid Glimelius), Swedish Society of Medicine (Ingrid Glimelius), Lions Research Cancer Fund (Ingrid Glimelius), Fru Berta Kamprad FBKS-2018-7-149 (Sara Ek) and the European Community’s Horizon 2020 Framework Programme for Research and Innovation (EU-H2020-MSCA-COFUND-2016-754299, Sara Ek).
Author contributions
Joana M. Rodrigues and Anna Nikkarinen were involved in the planning of the study, performed data and statistical analysis and wrote the manuscript. Peter Hollander and Anna Porwit performed the pathology review. Arne Kolstad, Riikka Rääty, Rose-Marie Amini and Mats Jerkeman were responsible for the collection of the material and/or clinical data. Caroline E. Weibull was involved in the statistical analysis. Sara Ek and Ingrid Glimelius were responsible for the planning of the study, analysis of the data and drafting the manuscript. All authors approved the final version of the manuscript.




