Venetoclax and decitabine for relapsed paediatric myelodysplastic syndrome-related acute myeloid leukaemia with complex aberrant karyotype after second stem cell transplantation
Paediatric myelodysplastic syndrome (MDS) is a rare clonal haematologic disorder characterised by cytopenia and ineffective, dysplastic haematopoiesis. Malignant transformation by predisposing clonal haematopoiesis to MDS-related AML (MDR-AML) is associated with a poor prognosis and requires allogeneic haematopoietic stem cell transplantation (alloHSCT).1, 2 Treatment of relapse after alloHSCT is usually not curative. Naik et al.3 report five-year survival rates of 10–35% after a second alloHSCT for relapsed malignant paediatric diseases, depending on various prognostic factors, including time of relapse after first alloHSCT and remission status at second alloHSCT. Subsequent relapses correlate with a worsening prognosis, given increased refractoriness to therapy through dynamic adaptive mechanisms of malignant cells.
For treatment-naive elderly AML patients ineligible for intensive chemotherapy, DiNardo et al.4 recently published promising data for the oral BCL-2 inhibitor venetoclax, combined with a hypomethylating agent (HMA), decitabine or azacitidine.
We report on a 16-year old male who was first diagnosed at the age of 14 years with MDS-RAEB-t (MDS-EB2 based on the 2016 revision to the WHO classification of MDS) with complex aberrant karyotype (del 5q, del 7q). Progression to MDR-AML under therapy with azacitidine was observed. He subsequently underwent alloHSCT from an unrelated donor (9/10 HLA match). Relapse after 17 months was treated with a second alloHSCT, with TCRα/β- and CD19-depleted peripheral blood stem cells (PBSC) from his HLA-haploidentical mother.5 Conditioning consisted of cytoreductive FLAMSA (fludarabine/amsacrine/cytarabine) and subsequent myeloablative conditioning (fludarabine/busulfan/clofarabine) (see Table I), based on Versluys et al.6 Engraftment of leucocytes, neutrophils and thrombocytes was observed on day +11. A pre-emptive donor lymphocyte infusion (DLI) was administered on day +75 for mixed bone marrow (BM) chimerism (Fig 1). Acute graft-versus-host disease (aGvHD) grade I (Skin 2, Gut 0, Liver 0) ensued and was treated with systemic corticosteroids (prednisolone 1 mg/kg/day) from day +91 to +95. The second relapse was diagnosed nine months after the second alloHSCT (day +272) on a routine BM aspirate with 40% myeloblasts and mixed donor chimerism. Palliative care was declined by the family owing to the patient’s excellent general condition. The relapse proved refractory to cytoreductive chemotherapy with cytarabine (two cycles, each 4 × 50 mg/m2 s.c.) in combination with three therapeutic DLIs in increasing doses. Donor chimerism decreased to 15%, with 55% myeloid blasts in peripheral blood (Fig 1).
| Medication | Total dose (mg) | Daily dose | Day |
|---|---|---|---|
| Fludarabine | 250·8 | 30 mg/m2 | –15 to –12 |
| Amsacrine | 836 | 100 mg/m2 | –15 to –12 |
| Cytarabine | 16 720 | 2000 mg/m2 | –15 to –12 |
| ATG | 2652 | 10 mg/kg | –11 to –9 |
| Fludarabine | 83·6 | 10 mg/m2 | –7 to –4 |
| Busulfan* | 919·36 | 4 × 0·8 mg/kg, total of 13 doses | –7 to –4 |
| Clofarabine | 250·8 | 30 mg/m2 | –7 to –4 |
- alloHSCT, allogeneic haematopoietic stem cell transplantation; ATG, anti-thyreoglobulin antibody; TDM, therapeutic drug monitoring.
- * TDM: tAUC = 88 448·5 ng × h/ml.
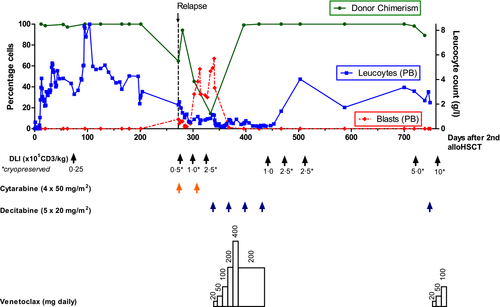
We decided to offer the patient an experimental, outpatient therapy of venetoclax and decitabine.4 The regimen consisted of four 28-day cycles of intravenous decitabine (days 1–5 of each cycle) and continuous oral venetoclax (beginning on day 1 of cycle 1, ending on day 28 of cycle 3). Venetoclax dose ramp-up was performed in accordance with manufacturer’s recommendations over five weeks. Prophylaxes with valaciclovir, trimethoprim/sulfamethoxazole and posaconazole were continued. Routine screening for CMV, EBV, adenovirus and aspergillus remained negative. One event of febrile neutropenia CTCAE grade 3 occurred during the first cycle. Remission was observed on BM aspirate after the second cycle with full donor chimerism. Three additional prophylactic DLIs were administered after completion of four cycles without signs of aGvHD. During therapy the patient remarkably completed his high-school diploma and began an apprenticeship. Complete remission (CR), as documented by repeated BM aspirates, persisted for 10 months off-therapy. Unfortunately, a subsequent molecular relapse was detected at 15 months post-relapse, which progressed to overt relapse with complex aberrant karyotype and mixed chimerism on BM analysis. Subsequently, the patient was restarted on venetoclax and decitabine with therapeutic DLIs planned for every four weeks in increasing doses.
Relapsed paediatric malignancies pose great medical and ethical challenges. Consecutive alloHSCTs may offer curative treatment for a very limited proportion of patients, but may cause unnecessary harm to the remainder.3 Recent data for treatment-naive, elderly AML patients with a combined chemotherapy consisting of venetoclax and HMAs have shown promising outcomes.4 The oral BCL-2 inhibitor venetoclax targets anti-apoptotic mechanisms in myeloid blasts, triggering a mitochondrial-dependent apoptotic pathway.7 HMAs, such as azacitidine or decitabine, or LDAC, constitute lower intensity treatments for patients ineligible for intensive chemotherapy, but are associated with low response rates when administered as monotherapy.4, 8 Alternatively, individualised targeted-therapy options for mutated NRAS (neuroblastoma RAS) would have been available for this patient. NRAS mutations are frequently described in AML patients9 and are also common in paediatric MDS.10 However, these results were available only after beginning therapy with venetoclax and decitabine. Numerous targeted therapies combined with venetoclax are currently under investigation and may lead to further treatment options in our patient.8
Gaut et al.11 recently reported their experience with venetoclax combination therapy in 14 adult patients with relapsed/refractory AML. Prolonged severe cytopenias were the main adverse effect. The results, with an overall response rate of 36%, no CR and a median overall survival of 4·7 months were not quite as positive as in our patient. A younger age is possibly a good prognostic factor, but other yet unidentified molecular disease characteristics may play a larger role.
Our patient exhibited prolonged neutropenia during venetoclax therapy, possibly enhanced by concomitant posaconazole administration.8, 12 It is debatable whether antifungal prophylaxis was necessary, as invasive fungal infections during venetoclax/HMA treatment are rare.13 Dose reduction by 50% of venetoclax was performed instead of the 75% reduction recommended by the manufacturer and literature,8, 12 with only moderate recovery of neutrophil count. Beneficial anti-leukaemic effects of increased venetoclax exposure are speculative; therapeutic drug monitoring (TDM) was not performed. Recent investigations show best responses after the first cycle of combination therapies with venetoclax.8 Therefore, venetoclax dose reductions or pauses for patients in CR have been recommended to reduce aplasia.8
With this report of prolonged CR in a patient relapsed after two alloHSCTs we hope to stimulate interest for venetoclax + HMA treatment for refractory or relapsed paediatric MDS/AML. Further studies are necessary to fully assess safety and efficacy and to establish which patients will benefit most.
Acknowledgement
The authors wish to thank the entirety of medical staff involved in this patient’s care.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
JR and MA wrote the manuscript. JR, SH, MK, AE, VB, IS, CK, TF and MA contributed to the patient’s medical care, edited and approved the manuscript.