High-level induction of fetal haemoglobin by pomalidomide in β-thalassaemia/HbE erythroid progenitor cells
Studies have shown that increased expression of fetal haemoglobin (HbF; α2γ2) can ameliorate red blood cell deficiencies in patients with β-thalassaemia and sickle cell disease (SCD).1-3 Pharmacological induction of HbF expression in β-thalassaemia has been investigated using several classes of small molecules,4 including 5-azacytidine,5 decitabine,6 hydroxyurea,7 LSD1 inhibitors (tranylcypromine and RN-1),8, 9 and short chain fatty acid derivatives.10, 11 Among these molecules, hydroxyurea is the only U.S. Food and Drug Administration (FDA) currently approved drug for the treatment of SCD and/or β-thalassaemia. However, hydroxyurea has shown modest and variable responses with potential myelosuppression in β-thalassaemia patients. Therefore, more robust and safer HbF therapeutics are highly desired.
Pomalidomide, an FDA-approved immunomodulatory drug for the treatment of multiple myeloma,12, 13 stimulates γ-globin mRNA and HbF expression in erythroid progenitor cells by downregulating factors involved in γ-globin repression, including BCL11A, SOX6, GATA1, KLF1 and LSD1.14-16 In addition, treatment of a humanized mouse model of SCD with pomalidomide induced comparable HbF expression to hydroxyurea, but without myelosuppressive effects.15
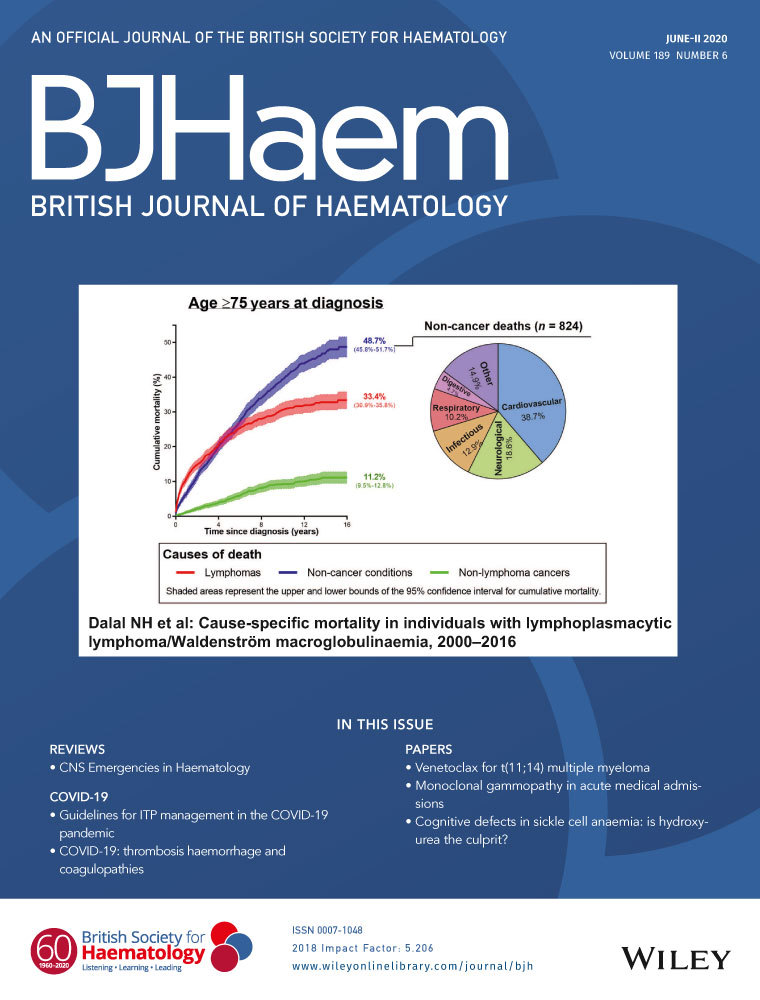
Here, we investigated the therapeutic potential of pomalidomide and its combined effects with other HbF inducers, including hydroxyurea, decitabine and RN-1, in erythroid progenitor cells from compound heterozygous β0-thalassaemia/HbE (HBB:c.79G>A) patients (Table SI) using a three-phase liquid culture system that supports terminal maturation of erythroid cells (Data S1 and Fig S1). Comparison of results using optimal conditions for each compound (Figs S2 and S3) revealed that pomalidomide was much more effective in inducing HbF expression than hydroxyurea, decitabine or RN-1 (Fig 1A,B). The greatest increase in HbF percentage from the baseline level was observed in pomalidomide-treated cells, achieving 25·6 ± 1·1% as determined by high-performance liquid chromatography (HPLC) (Fig 1A,B). β0-thalassaemia/HbE precursors from patients of different β0-thalassemic mutations (Table SI) showed similarly increased levels of HbF induction in response to pomalidomide treatment. This result suggested that deficient progenitors, regardless of specific β0-thalassemic mutation or baseline HbF level, are all susceptible to strong induction with pomalidomide (Fig 1A,B and Tables SI and SII). The percentage of cells expressing HbF (F cells) increased from 49·8 ± 4·7% for dimethyl sulfoxide (DMSO) controls to 60·6 ± 2·5% after pomalidomide treatment (Fig S4). By quantitative reverse transcription polymerase chain reaction (RT-PCR), we found that pomalidomide significantly increased γ-globin (HBG) mRNA expression, achieving a 2·3 ± 0·3-fold increase over control cells, with coincidentally diminished β-globin (HBB) expression, without significant change in α-globin (HBA) expression (Fig 1C).
To enhance the level of HbF induction, we investigated the effects of combined treatment of pomalidomide either with or without other pharmacological HbF inducers. The combination of pomalidomide and decitabine had an additive effect on induction, as shown by the differential HbF level (Δ%HbF = 36·7 ± 1·3) when compared to treatment with any single agent (Fig 1A,B). Hydroxyurea did not generate any additional increase in HbF when combined with pomalidomide. The combination of pomalidomide and RN-1 did increase the percentage of HbF (Fig 1A,B) and at the same time reduced HBA, HBB and HBG mRNA expression (Fig 1C), suggesting that this combination negatively affected total globin mRNA expression. Taken together, these results suggest that pomalidomide and decitabine act through independent pathways to induce, additively, high-level HbF expression, implying a cooperative therapeutic potential for the treatment of β-thalassaemia.
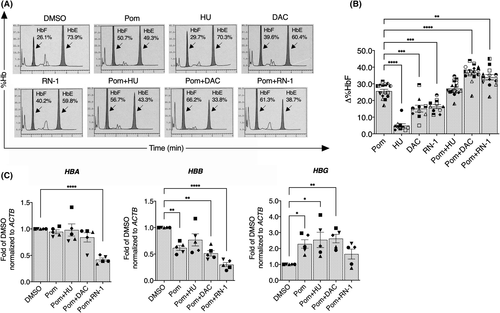
We next determined the cytotoxicity of treatments and found that pomalidomide did not significantly affect erythroid cell proliferation (Fig S5A) or viability (Fig S5B). However, pomalidomide plus decitabine showed a reduction in cell proliferation on day 12 of culture without affecting cell viability. Erythroid cell proliferation and viability were significantly reduced in cells exposed to pomalidomide plus RN-1 (Fig S5A,B), suggesting toxicity of the latter combination. Analysis of erythroid differentiation of cells treated with hydroxyurea or pomalidomide plus hydroxyurea was similar to that of DMSO-treated cells (Fig 2A,B), suggesting that these treatments did not affect erythroid terminal differentiation. We noted a trend towards increased differentiation of cells treated with pomalidomide, RN-1 and pomalidomide plus RN-1, compared with the controls. Interestingly, significantly accelerated erythroid differentiation was observed in decitabine alone and pomalidomide plus decitabine, as evidenced by elevated transferrin receptor (CD71)medium/(glycophorin A (GPA)high population and decreased CD71high/GPAhigh cells (Fig 2A,B). Similarly, modified Giemsa-stained cytospins showed an increased number of late-stage erythroblasts in cells exposed to decitabine alone and pomalidomide plus decitabine when compared to control cells, indicating a shift towards normal erythroid cell maturation (Fig 2C and Fig S1). These results suggested that the differentiation of β0-thalassaemia/HbE progenitor cells significantly improved after treatment with either decitabine alone or pomalidomide plus decitabine.
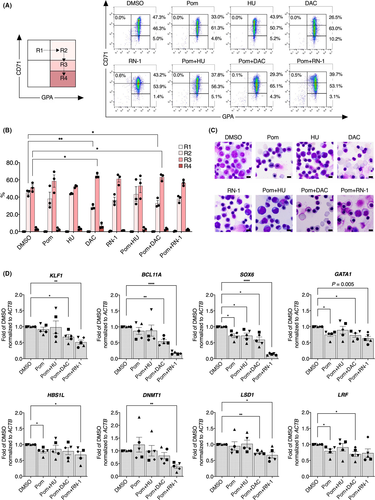
To investigate the effects of pomalidomide plus or minus these effectors on transcriptional regulation in β-thalassaemic erythroid progenitor cells, quantitative RT-PCR analyses revealed that one key γ-globin repressor mRNA, BCL11A, was only slightly reduced after treatment with pomalidomide or pomalidomide plus hydroxyurea. BCL11A was significantly downregulated (by 1·8- and 5·6-fold) after treatment with pomalidomide plus decitabine or pomalidomide plus RN-1 respectively (Fig 2D). Moreover, the expression of SOX6, GATA1, HBS1L and LRF were modestly but significantly downregulated by pomalidomide, whereas other erythroid regulators were unaffected (Fig 2D and Fig S6). In addition, combined pomalidomide and decitabine treatment, which showed additive effects on HbF induction, reduced the expression of KLF1, LSD1 and CHD4. The combination of pomalidomide plus RN-1 significantly affected the expression of several key regulators, including KLF1, SOX6, GATA1, HBS1L, DNMT1, LSD1, ID2, CHD4, FOXO3, NRF2 and MYB (Fig 2D and Fig S6), consistent with the fact that this same combination significantly reduced cell proliferation and viability (Fig S5). Taken together, these results indicate that the mechanisms of action of pomalidomide and several co-effectors in induction of HbF expression partly involve transcriptional regulation of key HbF repressors and/or co-repressors.
In summary, the present data show that pomalidomide is a potent HbF inducer and is more potent than hydroxyurea. The combination of pomalidomide and decitabine provide additive effects in inducing HbF expression in erythroid cells from β0-thalassaemia/HbE patients. Despite these promising results, it must be emphasized that the potential risks associated with the use of pomalidomide include developmental defects (if taken during pregnancy), thrombosis and pancytopenia,17 which are similar to the toxicities of the parental drugs, lenalidomide and thalidomide. Development of pomalidomide structural refinements or analogues with similar biological effects may lead to future, fully effective, reduced adverse effects and possible clinical application.
Acknowledgements
The authors would like to thank the patients and their families for their contributions to this study; and Thongperm Munkongdee, Nattrika Buasuwan, and Nurmeeha Hinna for their assistance with the DNA diagnosis for thalassaemia and haemoglobin analysis. The technical assistance of Greggory Myers is greatly appreciated. This work was supported by grants from Mahidol University, the Thailand Research Fund (MRG5680092) and the Office of the Higher Education Commission to N.J. P.P was supported by the Siriraj Graduate Scholarship.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
PK, TN, OS and NJ designed the research; PK, TN, PP and WK performed the experiments; PK, TN and NJ analysed data; DS, KP, SH and SF provided samples and resources; PK, JDE, and NJ wrote the manuscript; JDE, SH, SF, OS and NJ conceptualized the idea and supervised the project. All the authors read and approved the final manuscript.