Venous and arterial thrombosis in patients with haematological malignancy during treatment with ibrutinib
Ibrutinib, an irreversible inhibitor of Bruton tyrosine kinase (BTK), is approved for the treatment of multiple B-cell malignancies (Byrd et al, 2013; Treon et al, 2015; Noy et al, 2017). Ibrutinib also inhibits other tyrosine kinases which may be responsible for its well-established toxicities of atrial fibrillation (AF) and bleeding as well as increased risk of haemorrhage when combined with anticoagulation (AC) or antiplatelet agents (Byrd et al, 2013; Berglof et al, 2015; Lipsky et al, 2015). The population most likely to be exposed to ibrutinib is at higher risk for thrombosis given the presence of a maligiancy, such as chronic lymphocytic leukaemia (CLL), where the risk of venous thrombosis (VTE) was ~1·5 per 100 patients years, as well increased age where VTE risk is higher (Whittle et al, 2011; Heit, 2015; Simkovic et al, 2015). Additionally, arterial thrombosis risk also increases with age and in malignancy, and ibrutinib increases risk of AF, which may increase stroke (Leong et al, 2016; Wiczer et al, 2017). Therefore it is vital to understand the incidence of thrombosis in patients taking ibrutinib, which is presently unknown. To determine this we conducted a single-institution retrospective cohort study of patients taking ibrutinib as treatment for a haematologic malignancy.
We identified 565 patients who received ibrutinib. Details regarding methods are in the supplement along with statistical methods. The median age of these patients was 65 (range 23–98) years, 70·3% were men, 73·6% had CLL and 81% were treated through a clinical trial. Before the initiation of ibrutinib, 78 (13·8%) had a history of 99 VTE episodes. The median time from the last VTE to ibrutinib initiation was 1·54 years (range 0–36·7). Detailed chohort characteristcs are in Table SI.
Median ibrutinib exposure was 2·39 (range 0–7·36) years per patient. At any time concurrently with ibrutinib, 224 (39·6%) patients received anti-platelet agents and 47 (8·3%) patients received AC. Median overlap of AC and ibrutinib was 59·5 days (range 2–977 days).
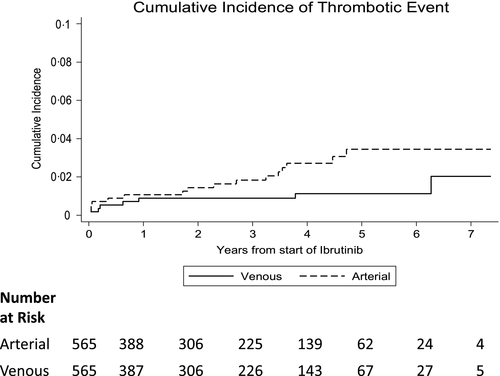
During ibrutinib treatment, 22 of 565 (3·9%) patients experienced 24 acute thrombotic events, including eight venous and 16 arterial (Table 1). The incidences of venous and arterial thrombosis were 0·6 [95% confidence interval (CI): 0·2–1.1] and 1·1 per 100 person-years, respectively (95% CI: 0·6–1·8). After starting ibrutinib, VTE developed at a median of 7·5 (range 0·5–75·3) and arterial thrombosis developed at a median of 24·6 (range 0·4–56·6) months (Fig 1).
| Types of thrombosis | |
| Thrombus type | N = 24* |
| Arterial, n (%) | 16 (66·7) |
| Venous, n (%) | 8 (33·3) |
| Arterial thrombus type | N = 16 |
| MI, n (%) | 3 (18·8) |
| CVA, n (%) | 6 (37·5) |
| Peripheral arterial, n (%) | 1 (6·3) |
| TIA, n (%) | 4 (25·0) |
| Intracardiac, n (%) | 2 (12·5) |
| Venous thrombus type | N = 8 |
| DVT, n (%) | 7 (87·5) |
| Retinal vein occlusion | 1 (12·5) |
| DVT location | N = 7 |
| Upper extremity, n (%) | 3 (42·9) |
| Proximal lower extremity, n (%) | 2 (28·6) |
| Distal lower extremity, n (%) | 1 (14·3) |
| Unknown lower extremity, n (%) | 1 (14·3) |
| Thrombosis management | |
| Arterial thrombus treatment | N = 14** |
| Aspirin, n (%) | 5 (35·7) |
| Clopidogrel, n (%) | 1 (7·1) |
| Aspirin + Clopidogrel, n (%) | 1 (7·1) |
| Aspirin + DOAC, n (%) | 2 (14·3) |
| DOAC, n (%) | 3 (21·4) |
| No pharmacologic agent, n (%) | 2 (14·3) |
| Venous thrombus treatment | N = 8 |
| Aspirin, n (%) | 1 (12·5) |
| LMWH, n (%) | 2 (25·0) |
| Warfarin, n (%) | 1 (12·5) |
| Fondaparinux, n (%) | 1 (12·5) |
| DOAC, n (%) | 1 (12·5) |
| No pharmacologic agent, n (%) | 2 (25·0) |
| Ibrutinib management after venous thrombosis | N = 8 |
| Continue, n (%) | 3 (37·5) |
| Hold, n (%)# | 2 (25·0) |
| Discontinue, n (%) | 3 (37·5) |
| Ibrutinib management after arterial thrombosis | N = 16 |
| Continue, n (%) | 3 (18·8) |
| Hold, n (%)# | 7 (43·8) |
| Discontinue, n (%) | 6 (37·5) |
- CVA, cerebrovascular accident; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; LMWH, low molecular weight heparin; MI, myocardial infarction; TIA, transient ischaemic attack.
- * Two patients had two thrombotic events.
- ** Excluded two patients who died within 7 days of thrombosis.
- # For both venous and arterial events, the median number of days ibrutinib was held was 20 days (range 3–93).
Of the VTE, 87·5% (7/8) were deep vein thrombosis (DVT). The majority were associated with provoking factors - three patients had a central line and six patients were immobile and/or hospitalised. Five of eight (62·5%) VTE were treated with AC, which was continued for a median of 29 days (range 14–156).
Of the 16 arterial thrombosis events, the majority were acute cerebrovascular accidents (CVA) (6/16, 37·5%), with AF probably contributing to four events. The most common treatment was aspirin (5/14, 35·7%). Five patients (5/14, 35·7%) were treated with a direct oral anticoagulant (DOAC) with or without aspirin: two with intracardiac thrombus, two with transient ischaemic attack and one with peripheral vascular disease.
Following a thrombotic event, patients were evaluated for recurrent thrombosis and bleeding. There were nine bleeding events: four major and five minor (Table SII). Three patients died due to bleeding: two after haemorrhagic conversion of a CVA and one after a motor vehicle accident. Platelet counts at the time of the central nervous system haemorrhage were 34 × 109/l and 42 × 109/l. One patient developed a recurrent central line-associated thrombosis while on ibrutinib and AC.
Univariable analysis was performed to determine the factors associated with thrombosis during ibrutinib treatment. This showed that age and prior venous and arterial thrombosis were associated with increased risk of VTE (Table SIII). Longer duration of malignancy and age were associated with arterial thrombosis.
Our data supported that, in patients treated with ibrutinib, the rate of thrombosis was low (3·9%) and the cumulative incidence of VTE was lower than expected as compared to historical controls (0·6 per 100 person-years in our study compared to 1·5 per 100 person-years in prior studies of CLL patients) (Whittle et al, 2011; Simkovic et al, 2015)’. However, many patients in our study received concomitant antiplatelet agents or AC (39·6% and 8·3%, respectively), which may have reduced the risk of thrombosis even though the median overlap was short.
The incidence of arterial thrombosis was similarly low [1·1 per 100 person-years (95% CI: 0·6–1·8)] in a patient population at high risk for arterial disease. The low incidence of artieral thrombosis may have been because of ibrutinib's inhibition of BTK and Tec in platelets as an anti-platelet effect, which are proposed mechanisms for its bleeding toxicity (Byrd et al, 2013; Berglof et al, 2015; Lipsky et al, 2015).
Following a thrombotic event, significantly more bleeding complications (N = 9) occurred than recurrent thrombosis (N = 1) despite the short median duration of AC (29 days). At the time of the bleeding event, the majority of patients were taking ibrutinib with anti-platelet agents or AC. Major bleeding was rare and usually resulted from a secondary cause (Table SII).
There are several limitations to our study including its retrospective design and we may have underestimated the thrombosis incidence as we were reliant on physician documentation. Similarly, our study may have underestimated bleeding. Despite these limitations, to our knowledge, this is the first report of thrombosis incidence and outcomes of patients on ibrutinib. It reflects detailed information on a large cohort of patients, including patients outside of clinical trials and with a variety of malignancies.
Our study found a low incidence of thrombotic events, especially VTE, in patients taking ibrutinib. Prior history of thrombosis and older age were factors that increased the risk of VTE. The majority who developed a thrombosis while on ibrutinib were started on anti-platelet agents and/or AC, but many experienced bleeding events and recurrent thrombosis was rare. This analysis may help physicians consider how ibrutinib changes the risk-benefit ratio of ongoing AC and/or anti-platelet agents.
Acknowledgements
The authors would like to thank the Ohio State University Division of Hematology for support of this project. They would also like to acknowledge their colleagues who provided the clinical care for the included patients. Research reported in this publication was supported in part by the Ohio State University Comprehensive Cancer Center and the National Institutes of Health under grant number P30 CA016058, R35 CA197734 (JCB), R01 CA177292 (JW, JCB).
Authorship contributions
E.M.K. collected data, analysed results, and wrote the paper; Q.Z. performed statistical analysis and created the tables and figues; S.B., J.B., and J.W. reviewed the manusctipt; J.H., L.O., and T.W collected data. F.T.A helped with study design. K.A.R and T.W. assisted with data interpretation, literature searches, writing the paper and study design.
Conflict of interest
Ms. Zhao, and Drs Hirsch, Byrd, Ooka, Kander and Wiczer have no relevant conflicts of interest. Dr. Rogers receives research funds from Abbvie and Genetech and consulted for Acerta. Dr. Wang consulted for Daiichi Sankyo and Pfizer. Dr. Woyach receives research funds from Abbvie, Janssen, Loxo, Pharmacyclics, Morphosys, and Karyopharm and has consulted for Pharmacyclics and Janssen. Dr. Bhat served on the Speakers` Bureau for Pharmacyclics and Janssen. Dr. Awan has served on advisory boards for Gilead, Pharmacyclics, Abbvie, Janssen, AstraZeneca, Genentech, Sunesis; received research funding from Pharmacyclics; and served on the speakers bureau for AstraZeneca and Abbvie.