Development of artificial bone marrow fibre scaffolds to study resistance to anti-leukaemia agents
The mechanisms for resistance in chronic myeloid leukaemia (CML) patients with no BCR-ABL1 kinase domain mutations are unknown (Apperley, 2007). Similar situations can be observed in other myeloid malignancies, such as acute myeloid leukaemia (AML), in which patients may develop resistance to agents targeting molecular drivers, such as aberration of the FLT3 gene, via mechanisms that are equally poorly understood (Parmar et al, 2011). Many lines of evidence support the presence of persistent low-levels of quiescent leukaemia stem cells residing in the bone marrow (BM) niche and surviving independent of their tyrosine kinase activity, possibly through interaction with the microenvironment. There is also evidence that the interaction of leukaemia cells with an artificial scaffold in three-dimensional (3D) culture causes resistance to chemotherapeutic agents (Aljitawi et al, 2014). Two-dimensional (2D) cell-based assays have limited value in predicting long-term clinical response to anti-cancer drugs (Kunz-Schughart et al, 2004), mainly due to absence of the extracellular matrix (Cox & Erler, 2011).
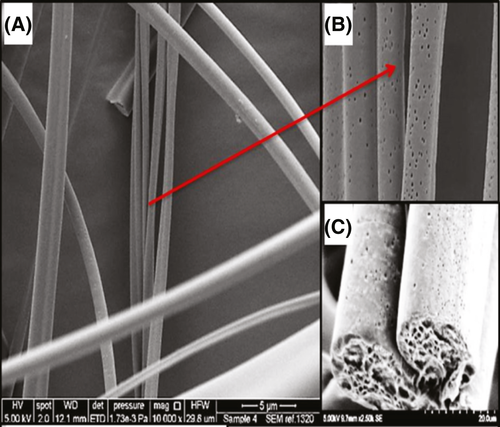
To address this experimental limitation, attempts have been made to produce 3D cultures (Imamura et al, 2015). Polymeric fibres have been applied extensively in the manufacture of scaffolds because of their unique characteristics, such as high surface area to volume ratio, high rate of absorption and low density (Wu et al, 2017). We hypothesized that a 3D culture using a fibre-based synthetic scaffold designed to mimic the BM microenvironment might provide an optimal microenvironment for proliferation of leukaemia cells. Pressurized gyration is a simple and cost-effective technique which can generate fibres at a high production rate with control of surface features such as roughness and porosity (Illangakoon et al, 2016); therefore it was used for the development of a 3D Poly(methylmethacrylate)-hydroxyapatite (PMMA-HA) fibre scaffold in this study (Data S1). Scanning electron microscopy (SEM) images of the PMMA-HA fibres reveal a rough surface containing nanopores and HA nanoparticles (Figs 1 and S1). Composite fibres were continuous and cylindrical in shape without any bead-on-string morphology indicating that the solution and process parameters were correctly tuned during the gyration process (Figs 1 and S2). To demonstrate that these fibres are not toxic to cells, leukaemia cells from an AML patient were cultured in the PMMA-HA scaffolds (see Data S1). Microscopic observation revealed that these cells proliferated in the presence of the scaffold and concentrated around the fibres (Figure S3).
To investigate whether this culture was capable of recapitulating in vivo imatinib resistance, K562 cells were cultured in the presence or absence of the lowest inhibitory dose of imatinib (0·5 μmol/l) in 2D and 3D culture for 72 h followed by viability and proliferation assessment (see Data S1). Imatinib-induced inhibition was more significant in 2D (70% reduction) compared to 3D culture (56% reduction; P = 0·04) (Fig 2A). To investigate if the protective effect of PMMA-HA-based 3D culture is specific against imatinib; the response of other leukaemia cells to antileukaemia agents was investigated. More live AML-derived HL60 cells were observed in the 3D culture after treatment with 10 μM doxorubicin compared to 2D culture (8% vs. 4%, respectively). This difference did not reach significance (P = 0·07), but suggested a trend toward more protection by the scaffold (Fig 2B).
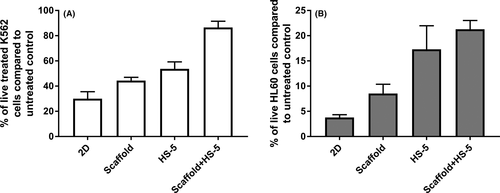
To further mimic the BM, which has cellular and matrix components, the PMMA-HA-based culture was modified via the addition of HS-5 cells, a stromal cell line derived from human BM (see Data S1). The co-culture of HS-5 and K562 cells has been reported to reduce the sensitivity of K562 cells to the inhibitory effect of imatinib due to the release of cytokines activating alternative survival pathways (Eiring et al, 2014). We found a reduced sensitivity of co-cultured K562 cells to imatinib compared to those cultured alone (47% reduction vs. 70% reduction; P = 0·006) (Fig 2A). Similarly, co-culture of HL60 cells with HS-5 showed a trend toward reduced doxorubicin sensitivity, although this did not reach significance (P = 0·055; Fig 2B). When added to the PMMA-HA-based culture, HS-5 cells appeared to be attracted to the scaffold, as observed with K562 (Figure S4A, S4B) and AML cells. We then investigated whether the combination of scaffold with HS-5 stromal cells had any additive effect on the sensitivity of the K562 and HL60 cells to antileukaemia agents. HL60 or K562 were added to the HS-5 3D culture followed by the addition of doxorubicin or imatinib 24 h later, and live cells were counted 72 h after adding the drugs. The combination of HS-5 cells and scaffold further reduced the sensitivity of K562 cells to imatinib compared to co-culture with HS-5 in 2D (30% vs. 47% reduction; P = 0·006) and to 3D scaffold without HS-5 (30% vs. 44% reduction; P = 0·008). The same experiment with HL60 cells treated with doxorubicin showed a similar trend although this did not reach significance (Fig 2B).
In summary, the proliferation and concentration of primary AML, K562, HL60 and HS-5 cells around the fibres supported the suitability of this matrix for studying leukaemia cells. The induced relative resistance to either imatinib or doxorubicin supported the notion that the 3D structure of the BM protects cells against chemotherapeutic agents. The effect might be because the cells have not had the same degree of exposure to the drugs due to being shielded in the fibres, where there may be a lower drug concentration, or alternatively because attaching to the fibres reduces cells’ dependence on the targeted oncoprotein. Combining the PMMA-HA with HS-5 cells enhanced similarity to the BM microenvironment by encompassing all of its basic components: scaffold, stromal cells and cytokines, the latter secreted by HS-5 (Traer et al, 2012). This 3D model, which we termed advanced PMMA-HA 3D culture, fostered an increased level of resistance to imatinib compared to either 2D culture or PMMA-HA culture, with the stromal cells contributing a larger proportion of the phenotype. In this study, we have demonstrated that advanced PMMA-HA-based 3D culture recapitulates the resistance to targeted therapy observed in a subset of leukaemia cells in vivo. The system may therefore permit the design of therapies capable of circumventing the protective role of the microenvironment, allowing complete, rather than transient cure from disease.
Author contributions
MK performed the research and analysed the data, EI, SC and AGR contributed essential reagents and tools, ME and JSK designed the experiments and wrote the paper.
Acknowledgements
The gyration work in the department of mechanical engineering, University College London was supported by EPSRC grants (EP/L 023059/1, EP/N 034228/1). The biological study was supported with fund from the Friends of Hammersmith Hospital, Kay Kendal Leukaemia Fund and Leuka. For the microscopy images, we acknowledge the Facility for Imaging by Light Microscopy (FILM) at Imperial College London (Hammersmith Campus) that is part-supported by funding from the Welcome Trust (grant 104931/Z/14/Z) and BBSRC (grant BB/L015129/1). We would like to thank Tom Gregory for his assistance with SEM imaging.




