SETBP1 mutations in juvenile myelomonocytic leukaemia and myelodysplastic syndrome but not in paediatric acute myeloid leukaemia
Juvenile myelomonocytic leukaemia (JMML) is a rare myeloproliferative disorder that is characterized by excessive myelomonocytic proliferation (Loh, 2011). Gene mutations in the components of the RAS signalling pathways are a hallmark of JMML and are considered to be central to the pathogenesis of JMML. Mutations in NRAS, KRAS, PTPN11, NF1, and CBL genes are found in approximately 75–85% of patients with JMML and are implicated in the aberrant RAS signalling (Loh, 2011). These mutations are also associated with congenital abnormalities, such as cardio–facio–cutaneous syndrome (KRAS), Noonan syndrome (PTPN11), neurofibromatosis (NF1), and Noonan-like syndrome (CBL). However, no other mutations have been identified in the remaining approximately 20% of patients with JMML.
In this regard, massively parallel sequencing technology has recently identified recurrent somatic mutations in SETBP1 in atypical chronic myeloid leukaemia (aCML) (Piazza et al, 2012). Of the 70 patients with aCML that were examined, 17 (24%) were found to carry SETBP1 mutations. These mutations clustered between codons 858 and 871, all located in the SKI-homologous region of SETBP1. Identical nucleotide alterations have been reported in Schinzel–Giedion syndrome (Hoischen et al, 2010), a rare congenital disorder that is characterized by severe mental retardation, distinctive facial features, and higher than normal prevalence of tumours, notably neuroepithelial neoplasia (Schinzel & Giedion, 1978). This report prompted us to search for possible SETBP1 mutations in JMML or other paediatric haematological malignancies.
To assess the clinical significance of SETBP1 mutations in paediatric leukaemias, we analysed a total of 414 patients with paediatric leukaemia/myelodysplastic syndrome (MDS) that comprised 42 patients with primary JMML, 24 with MDS, 22 with therapy-related leukaemia, 68 with infant acute lymphoblastic leukaemia (ALL), and 258 with de novo acute myeloid leukaemia (AML), including 10 patients with acute promyelocytic leukaemia (APL) and 22 with acute megakaryoblastic leukaemia (AMKL). The median age at diagnosis of JMML was 1 year and 10 months (range, 2 months to 8 years and 4 months), with 27 males and 15 females. MDS included 9 patients with refractory anaemia (RA), 14 with RA with an excess of blasts, and 1 with secondary MDS. The genomic region of the SETBP1 gene, containing codons 858–871 with the mutation hotspots D868 and G870 in the SKI-homologous region, was amplified using polymerase chain reaction (PCR) with the following primer sequences: forward, 5′-ACCAAAACCCAAAAGGGAAT-3′; reverse, 5′-CGGTTTTGCAGGCTTTTC-3′. Purified PCR products were sequenced using an ABI PRISM 3130 Genetic Analyser (Applied Biosystems, Branchburg, NJ). Mutations in RAS, PTPN11, and CBL have been previously reported in JMML (Shiba et al, 2010). The present study adhered to the principles of the Helsinki Declaration and was conducted under the regulations outlined by the Ethics Board of Gunma Children's Medical Centre.
SETBP1 mutations were found in 2 of the 42 patients with JMML (4·8%; Gly870Arg in JMML 2, Ser869Arg in JMML 24) and one of the 24 patients with MDS (4·2%; Ile871Thr in MDS 3) but not in the 22 patients with secondary AML, 68 with infant ALL, or 258 with de novo paediatric AML, including 10 patients with APL and 22 with AMKL (Fig 1A). The origin of the mutations was not determined due to the lack of appropriate normal tissue samples. In all 3 patients with SETBP1 mutations, a chromatogram exclusively showed a mutated sequence, indicating that the mutations were heterozygous (Fig 1A). Although one of the 2 JMML patients with an SETBP1 mutation survived after unrelated cord blood transplantation, the other died following relapse 4 months after undergoing related peripheral blood stem cell transplantation (Table 1). In contrast, the MDS patient who had an SETBP1 mutation was initially diagnosed with neuroblastoma at the age of 6 years. He was subsequently treated with chemotherapy and autologous bone marrow transplantation and achieved complete remission (CR). However, 3 years after the initial diagnosis, blast cells appeared in his peripheral blood and he was diagnosed with secondary MDS. Chromosomal analysis of the bone marrow cells revealed 45, XY, −15, der(7)t(7;15)(p13;q15), add(18)(q21) and add(20)(p13). He received chemotherapy with etoposide and cytarabine; however, he did not achieve CR. He died of haemorrhagic shock 18 months after being diagnosed with secondary MDS.
| Patient | Sex | Age | WBC (×109/l) | Karyotype | Nucleotide change | Amino acid change | SCT | Relapse | Survival (months) | Other mutations |
|---|---|---|---|---|---|---|---|---|---|---|
| JMML-2 | F | 24 months | 39·9 | 46XX | 2608A > C | Gly870Arg | U-CBT | – | 182+ | PTPN11 |
| JMML-24 | M | 26 months | 24·5 | 46XY, −7 | 2605G > C | Ser869Arg | allo-PBSCT | + | 6a | NRAS |
| MDS-3 | M | 12 years | 6·3 | 45XY, −15, der(7)t(7;15)(p13;q15), add(18)(q21), add(20)(p13) | 2612T > C | Ile871Thr | – | – | 18b | – |
- F, female; M, male; Gly, glycine; Arg, arginine; Ser, serine; Ile, isoleucine; Thr, threonine; SCT, stem cell transplantation; U-CBT, unrelated cord blood transplantation; allo-PBSCT, allogeneic peripheral blood stem cell transplantation; +, alive.
- a Died of relapse 6 month after initial diagnosis.
- b Died of haemorrhage shock 18 months after diagnosed with secondary MDS.
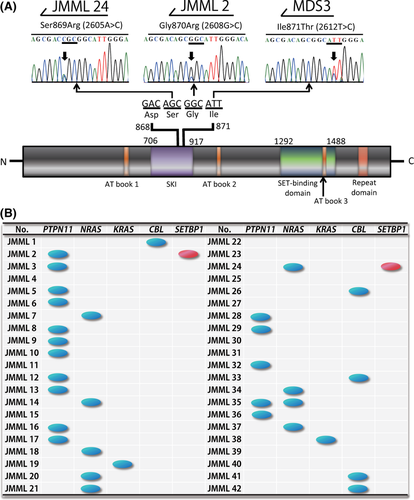
Mutations in NRAS, KRAS, PTPN11 and CBL genes were found in 21%, 4·8%, 38% and 12% of patients with JMML respectively, in our study (Fig 1B) (Shiba et al, 2010). Although almost all of the NRAS, KRAS, PTPN11 and CBL mutations occurred in a mutually exclusive manner, SETBP1 mutations were found in patients with PTPN11 or NRAS mutations (Table 1 and Fig 1B). This finding suggests that both gene mutations associated with the RAS pathway and SETBP1 mutations can cooperate in the pathogenesis of JMML.
High levels of SETBP1 expression have been described in elderly patients with AML (Cristobal et al, 2010), and SETBP1 has been identified in a specific paediatric T-cell ALL as a chromosomal translocation partner of NUP98 (Panagopoulos et al, 2001). SETBP1 has been reported to promote the self-renewal of murine myeloid progenitors via activation of HOXA9 and HOXA10 (Oakley et al, 2012). The patients with an SETBP1 mutation had a worse prognosis and presented higher white blood cell counts at diagnosis and also exhibited higher amounts of SETBP1 and SET protein, lower protein phosphatase 2A activity, and higher proliferation rates than those expressing the wild-type protein (Piazza et al, 2012). Although our cohort is too small to arrive at conclusions regarding the prognosis of patients with SETBP1 mutations, mutated SETBP1 indeed plays an essential role in the pathogenic mechanism in haematological malignancies.
In summary, SETBP1 mutations were found in 4·8% of patients with JMML in this study, similar to the frequency reported previously for patients with chronic myelomonocytic leukaemia [3·7% (3/82) and 6·2% (12/195)] (Piazza et al, 2012; Damm et al, 2013) and JMML [7·6% (7/92)] (Sakaguchi et al, 2013). Our analysis of 414 patients with JMML or other haematological malignancies suggests that mutations of SETBP1 may have some role in the pathogenesis of JMML and MDS but not in AML or infant ALL, although further evaluations are required.
Acknowledgements
The authors would like to thank Shinji Mochizuki, M.D., Division of Haematology/Oncology, Saitama Children's Medical Centre, Junko Takita, M.D., Department of Paediatrics, Graduate School of Medicine, University of Tokyo, and Keitaro Fukushima, M.D., Department of Paediatrics, Dokkyo Medical University, for providing the JMML samples. We also thank Ms. Yuki Hoshino, for her excellent technical assistance. This work was supported by a grant for Cancer Research, and a grant for Research on Children and Families from the Ministry of Health, Labour, and Welfare of Japan, a Grant-in-Aid for Scientific Research (B, C) and Exploratory Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by a Research grant for Gunma Prefectural Hospitals.
Authors contributions
Y.H. designed the study. K.K., M.Sotomatsu, M.Sako, and E.I. provided critical reagents and samples. N.S., K.O., and M.P. performed the experiments. E.I. and H.A. supervised the work. N.S., K.O., and M.P. analysed the results. N.S. and Y.H. wrote the paper and all the authors critically reviewed and revised it.
Conflict of interest
The authors declare no conflict of interest.




