Guideline on the management of primary resistant and relapsed classical Hodgkin lymphoma
1. Scope
The objective of this guideline is to aid clinicians in deciding which patients with primary refractory or relapsed Hodgkin lymphoma (HL) should receive salvage therapy with a view to autologous stem cell transplantation (ASCT); what response is adequate to allow ASCT and how to determine this; what is the role of radiotherapy in patient management; and what is the best management of patients unsuitable for autologous transplantation.
2. Methodology
The production of these guidelines involved the following steps:
- Establishment of a working group comprising experts in the field followed by literature review to 1 Feb 2013 including Medline, Pubmed and the Cochrane reviews database, using 1970 as a start date and the keywords: Hodgkin lymphoma, relapse, refractory, resistant, transplantation, PET, prognosis. In view of the paucity of phase III trials, all series excluding only those that were case reports were reviewed.
- The GRADE nomenclature was used to evaluate levels of evidence and to assess the strength of recommendations. See Appendix 1.
- Development of key recommendations was based on best available evidence. Due to the paucity of randomized studies the majority of recommendations are based on literature review and a consensus of expert opinion.
- Initial review of the manuscript was performed by the British Committee for Standards in Haematology (BCSH) Haem-Onc Task Force, and the British Society of Blood and Marrow Transplantation (BSBMT) executive committee.
- Final Review by the sounding board of the British Society for Haematology (BSH).
3. Background
Patients with primary resistant (progression or non-response during induction treatment or within 90 days of completion) or relapsed HL represent a relatively small but increasingly challenging population. The majority have classical HL. Although patients with nodular lymphocyte predominant HL (NLPHL) may be managed according to the same algorithms, some of these patients have a chronic indolent course and may not require such aggressive treatment (their management is subject to a separate BCSH guideline, currently in preparation). Repeat biopsy is generally recommended in patients thought to have relapsed, and should be considered in those who have residual fluorodeoxyglucose (FDG)-avid lesions post-therapy. This is important in order to confirm that there is no change in histology and to ensure that abnormalities on PET/computerized tomography (CT) imaging represent active disease. In some patients lesions can be difficult to access or yield non-diagnostic material despite multiple biopsies. In such cases identification of progression on serial imaging together with the presence of symptoms will increase confidence that such abnormalities truly represent disease, although it is acknowledged that, in some cases, salvage therapy will be warranted in the absence of histological proof or radiological progression.
4. Prognostic models
Prognostic models for de novo HL are well established for both early and late stage HL. However, prognostic models are less well defined for relapsed or refractory disease. Furthermore, as primary therapies become more effective and the numbers of treatment failures are reduced, it is likely that the cohort identified as either resistant or relapsed will become an increasingly poor prognostic group and that the relevance of previously identified prognostic factors will require re-evaluation. For example, the intensity of first-line therapy has an important impact on the outcome of salvage therapies, with evidence that patients receiving BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone) are more difficult to successfully salvage and rescue with high dose therapy than those relapsing after ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) (Josting et al, 2010). It is notable that a number of biological and pathological factors have been shown to correlate with prognosis, largely when assessed at diagnosis. (e.g. number of infiltrating CD68+ macrophages). Their independent importance has not been proven in large data sets, or specifically in the setting of relapsed or refractory disease.
4.1 Primary resistant disease
Primary resistance is generally considered, in itself, to be a poor prognostic marker (Longo et al, 1992; Bonfante et al, 1997; Moskowitz et al, 2001), and only limited models exist to distinguish one refractory patient from another. Clearly in this situation performance status and ability to tolerate intensive chemotherapy and ASCT will impact on survival (Josting et al, 2000; Ferme et al, 2002). Virtually no patients with primary resistant disease survive more than 8 years using conventional chemotherapy alone (0–8%)(Longo et al, 1992; Bonfante et al, 1997), whilst the projected 20-year survivals for those with early relapse (<12 months from primary therapy) or late relapse (>12 months from primary therapy) were previously estimated to be 11% and 22%, respectively, in the era of less intensive induction regimens (Longo et al, 1992). Whilst outcomes in those with primary progressive disease may appear reasonable following ASCT in some series (with a 5-year freedom from second failure (FF2F) of 42% reported by the German Hodgkin's Lymphoma study group (GHSG)), only a minority of such patients received ASCT (70/206, 33%) owing to rapidly progressive disease, therapy-related toxicity, insufficient stem cell harvest, or poor performance status (Josting et al, 2000). The 5-year FF2F for the entire cohort was only 17%. Poor performance status, age >50 years and failure to attain a temporary remission to first-line therapy were found to be poor prognostic factors in a multivariate analysis.
4.2 Relapsed disease
Patients with an initial period of remission have somewhat better outcomes. In a large retrospective analysis of 422 registry patients with relapsed HL performed by the GHSG (Josting et al, 2002a) the clinical factors predictive of worse 5-year FF2F were: time to relapse (3–12 months after completion of first treatment); stage at relapse (III or IV); and anaemia at relapse (<105 g/l in females and <120 g/l in males). The FF2F were 45%, 32% and 18% for those with scores of 0–1, 2 or 3 respectively. The prognostic score was predictive for patients who relapsed after radiotherapy, chemotherapy with conventional dose salvage, and chemotherapy with ASCT. Several other studies have shown that patients relapsing within 12 months of first line therapy have a poorer prognosis (Brice et al, 1997; Wheeler et al, 1997; Sureda et al, 2001); whilst 80% of those with a late relapse will achieve a second remission (Longo et al, 1992). Further factors found to have prognostic significance in some but not all studies include extranodal disease (Brice et al, 1997; Moskowitz et al, 2001) and the presence of B symptoms (Moskowitz et al, 2001). Using a 3-point scale based on (i) early relapse or primary refractory disease, (ii) extranodal disease, and (iii) presence of B symptoms at relapse, patients could be stratified with 5-year event-free survival (EFS) of only 27% and 10% in those with a score of 2 or 3 respectively (Moskowitz et al, 2001).
In summary, clinical factors prior to salvage that are most commonly identified as indicating poor prognosis include primary resistance, early relapse, disease bulk and/or stage plus allied systemic abnormalities. Performance status is also important.
4.3 Response to salvage
Response to salvage adds further discrimination in terms of prognosis, i.e., chemosensitivity, number of lines of salvage required, and status at transplant (Sureda et al, 2001). Refractoriness to salvage therapy predicts a very poor outcome. The more recent development of functional imaging modalities allows further refinement in this regard. The achievement of PET negativity following salvage therapy is a good prognostic indicator for outcome following ASCT, with 3–5 year progression-free survival (PFS) of >70% (Jabbour et al, 2007; Moskowitz et al, 2010; Smeltzer et al, 2011; Thomson et al, 2013). Residual FDG-PET-positive tumour uptake following salvage chemotherapy is associated with poor outcome following ASCT, even when ASCT is restricted to those achieving at least a partial response (PR) by conventional CT criteria (25–30% 3- to 5-year PFS) (Jabbour et al, 2007; Moskowitz et al, 2010). Thus whilst achievement of PR by CT criteria leads to recommendation for ASCT, 55–60% of such cases will have residual FDG-avid lesions following a single line of salvage (Moskowitz et al, 2010).
Recent reports have indicated potential for response-adapted management using PET to identify a further subgroup with potentially good outcomes post-ASCT, employing further intensive therapy for those patients with residual metabolic activity post-first line salvage. Conversion to PET-negative status post-second line salvage chemotherapy prior to ASCT was associated with a favourable outcome (EFS of >80%), equivalent to those who were PET-negative following first line salvage in those with only nodal disease, with somewhat inferior outcomes in those who achieve PET-negative status but with extranodal disease (Moskowitz et al, 2011). Thus, although PET status post-salvage probably overrides many previously identified prognostic factors to some degree, they may still have some independent impact and this issue requires further investigation. The possible role of a PET-based response-adjusted algorithm for directing patients to more experimental therapies is discussed further in the section on allogeneic transplantation.
Finally, whilst there is no a priori reason to indicate that PET responses following brentuximab vedotin will be in any way qualitatively different from those following other conventional salvage regimens, this question has yet to be addressed.
Recommendations
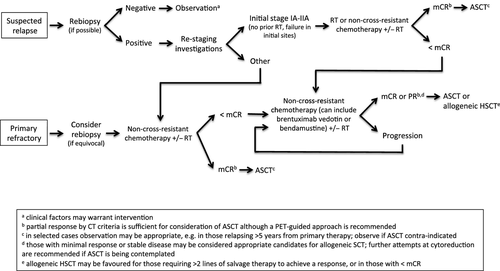
- Repeat biopsy is generally recommended in HL patients thought to have relapsed, and should be considered in those who have residual FDG-avid lesions post-therapy (1C) (Fig 1).
- PET-CT is the preferred restaging modality after salvage therapy (1B).
- The aim of salvage treatment should be to achieve an FDG-PET-negative remission (1B).
5. Salvage chemotherapy
5.1 First line salvage in patients eligible for high dose therapy
There are no randomized trials to compare the efficacy of chemotherapy regimens prior to ASCT. However, many single arm phase II trials have reported on the efficacy of a wide range of different regimens (Table 1). Overall response rates are reported as 70–90% and complete response rates (usually assessed with conventional CT scanning) as 20–55%. The confidence intervals reported by the trials frequently overlap and different patient populations were treated in these studies, making comparison of efficacy very difficult. Toxicity for the majority of regimens was mainly haematological, with gastrointestinal toxicity also a common feature of some regimens. Mortality from salvage therapy is low, as expected from the young age of the patients and associated lack of co-morbidities. However, the treatment-related mortality reported for the Dexa-BEAM (dexamethasone, carmustine, etoposide, cytarbine, melphaln) regimen was 5% in the GHSG/European Group for Blood and Marrow Transplantation (EBMT) ASCT phase III trial, although it is hard to know if this reflects an intrinsically greater toxicity for this regimen (Schmitz et al, 2002). Given that no recommendations can be made as to the most efficacious regimen, the decision should be tailored to individual patient needs (such as avoiding cisplatin in renal impairment or avoiding ifosfamide in patients at high risk of ifosfamide-induced encephalopathy) and using an established regimen which is familiar to the treating centre (Table 1).
| Regimen | ORR (%) | CRR (%) | References |
|---|---|---|---|
| ICE (ifosfamide, carboplatin, etoposide) | 88 | 26 | Moskowitz et al (2001) |
| IVE (ifosfamide, epirubicin, etoposide) | 85 | 37 | Proctor et al (2001) |
| MINE (mitoxantrone, ifosfamide, vinorelbine, etoposide) | 75 | 34 | Ferme et al (1995) |
| IVOx (ifosfamide, etoposide, oxaliplatin) | 76 | 32 | Sibon et al (2011) |
| IGEV (ifosfamide, gemcitabine, vinorelbine) | 81 | 54 | Santoro et al (2007) |
| GEM-P (gemcitabine, cisplatin, methylprednisolone) | 80 | 24 | Chau et al (2003) |
| GDP (gemcitabine, dexamethasone, cisplatin) | 70 | 52 | Baetz et al (2003) |
| GVD (gemcitabine, vinorelbine, liposomal doxorubicin) | 70 | 19 | Bartlett et al (2007) |
| Mini-BEAM (carmustine, etoposide, cytarabine, melphalan) | 84 | 32 | Colwill et al (1995) |
| DexaBEAM (dexamethasone, carmustine, etoposide, cytarabine, melphalan) | 81 | 27 | Schmitz et al (2002) |
| ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin) | 73 | 41 | Aparicio et al (1999) |
| ASHAP (doxorubicin, methylprednisolone, cytarabine, cisplatin) | 70 | 34 | Rodriguez et al (1999) |
| DHAP (dexamethasone, cytarabine, cisplatin) | 89 | 21 | Josting et al (2002b) |
| DHAOx (dexamethasone, cytarabine, oxaliplatin) | 74 | 43 | Rigacci et al (2010) |
| Bendamustine (NB. more heavily pre-treated cohort) | 53 | 33 | Moskowitz et al (2013) |
- ORR, overall response rate; CRR, complete response rate.
In addition to inducing remission, another important attribute of salvage regimens is a lack of toxicity to the stem cell compartment that would compromise mobilization and harvesting. Little comparative data exist on the impact of chemotherapy regimens on the success rate of stem cell collection. A retrospective comparison between collection after mini-BEAM (carmustine, etoposide, cytarabine, melphalan) and GDP (gemcitabine, dexamethasone, cisplatin) (Kuruvilla et al, 2006) appears to confirm that differences do probably exist. A collection of >5 × 106 CD34+ cells/kg was obtained in 97% of GDP-mobilized patients, but only 57% of mini-BEAM mobilized patients. A similar comparison in both HL and non-Hodgkin lymphoma (NHL) patients between IVE (ifosfamide, epirubicin, etoposide) and ICE (ifosfamide, carboplatin, etoposide) showed a collection of >5 × 106 CD34+ cells/kg was achieved in 72% and 51%, respectively, suggesting superiority for IVE (Fox et al, 2008), although it should be acknowledged that this cell dose is above that needed to support ASCT. Previous reports implicate prior exposure to agents that are toxic to stem cells as a risk factor for reduced stem/progenitor cell mobilization and subsequent engraftment. For example, a retrospective study identified, on multivariate analysis, that the number of courses of dexa-BEAM was the overriding factor affecting granulocyte-macrophage colony-forming unit (CFU-GM) collection from peripheral blood (although not from bone marrow)(Dreger et al, 1995). Two studies have identified the use of mini-BEAM as a risk factor for poor progenitor cell mobilization prior to ASCT (Watts et al, 1997; Weaver et al, 1998). Prior to stem cell collection it is therefore advised that regimens containing alkylating agents, such as melphalan and carmustine (e.g., mini-BEAM/dexa-BEAM), are avoided.
5.2 Intensive sequential strategies
A number of groups have investigated the role of intensive sequential chemotherapy regimens prior to ASCT, particularly in the higher risk cohorts, in order to try to improve outcomes (Tarella et al, 2003; Shea et al, 2009; Josting et al, 2010). To date any potential benefits have been offset by increased toxicities, with no evidence to suggest any significant overall benefits.
5.3 Second line salvage for patients eligible for high dose therapy
As previously discussed, patients who are PET-positive after first line salvage chemotherapy, as a group, have relatively poor outcomes. Those achieving PET-negative status following a second line of salvage may have outcomes that are similar to those achieving this status following a single line, at least for those with exclusively nodal disease, although this finding has only been reported in a single study and requires confirmation (Moskowitz et al, 2011). Nevertheless, it is recommended that patients should receive an alternative, non-cross reacting chemotherapy regimen in an attempt to achieve PET-negative status prior to ASCT. There are no data to support the choice of any particular regimen, though similar considerations apply regarding potential impact on mobilization. Alternatively, these patients may be considered for investigational strategies in the context of clinical trials.
There is no published evidence directly informing the question of how many cycles of each line of therapy should be administered before consideration of a switch to an alternative regimen. The consensus of the panel was that re-evaluation after 2 cycles of a multi-agent regimen was reasonable. Failure to demonstrate a significant improvement at this stage should prompt consideration of a switch to an alternative regimen. A third cycle should be considered in those responding well in order to try to achieve metabolic complete remission (CR). In the case of brentuximab vedotin, it is suggested that re-evaluation is undertaken after 3–4 cycles.
Very little data exist to guide treatment decisions in those patients who are refractory to first line salvage regimens, as defined by the failure to reach a partial remission. A proportion of patients do appear to benefit from second line salvage regimens. For example, in a small series of patients who were refractory to DHAP (dexamethasone, cytarabine, cisplatin) as initial chemotherapy, the administration of mini-BEAM second line resulted in a 59% overall response rate with some patients proceeding to ASCT (Stewart et al, 1991). Follow up post-ASCT was too short to draw conclusions as to the curative potential of this approach. A more recent retrospective study reported on 19 patients who had not responded to GDP first line salvage and went on to receive mini-BEAM. Six patients (32%) responded and nine proceeded to ASCT. Of these nine, seven relapsed, suggesting that with current salvage regimens, the outcome for refractory disease is very poor when using an alternative standard chemotherapy regimen (Villa et al, 2012). Finally, 12/19 (63%) patients refractory to at least one line of salvage (ESHAP; etoposide, methylprednisolone, cytarabine, cisplatin) responded to mini-BEAM in another retrospective study (Moore et al, 2012). Whilst 2-year PFS following consolidation with stem cell transplantation was a more encouraging 58% in this study, the vast majority underwent allogeneic rather than autologous transplant procedures (see below).
Alternative salvage agents include the anti-CD30 immunoconjugate brentuximab vedotin and bendamustine. The majority of data using brentuximab come from the pivotal phase II study in which patients were only eligible if they had relapsed following ASCT (Younes et al, 2012). The overall response rate was 75% with a 34% complete remission rate. Limited experience from small case series of up to 20 transplant-naïve patients has been published more recently. Overall response rates vary from 30 to 58% in ‘refractory’ patients (Forero-Torres et al, 2012; Gibb et al, 2013; Sasse et al, 2013). Two recent studies reported response rates of 53–58% in patients receiving bendamustine (Corazzelli et al, 2013; Moskowitz et al, 2013). Although most patients had received a prior ASCT, response was not influenced by chemosensitivity to previous line of treatment, suggesting this may be a useful second line salvage option.
5.4 Salvage chemotherapy for patients not eligible for high dose therapy
No prospective studies have specifically addressed the efficacy of second line chemotherapy alone (or chemotherapy combined with radiotherapy) in relapsed HL in patients not eligible for stem cell transplantation. In the randomized GHSG/EBMT study, Schmitz et al (2002) reported a 3-year freedom from treatment failure (FFTF) of 34% for those patients randomized to four courses of dexa-BEAM without ASCT. Outcome was better for those who relapsed late, defined as 12 months or more after initial therapy, with 3-year FFTF of 44% compared with 12% for those who relapsed between 3 and 12 months after initial treatment. Important caveats include the fact that the trial did not include patients with primary refractory disease, and that patients were only eligible for randomization if they achieved at least a PR by CT criteria, so this is a relatively highly selected group. Linch et al (1993) reported a 10% 3-year EFS for patients randomized to mini-BEAM alone but this was a significantly smaller study so stratification according to duration of first response is difficult. Again, patients in this study were, by definition, eligible for ASCT. Furthermore, both studies incorporated salvage regimens that have significant associated toxicities and may be poorly tolerated by older patients with co-morbidities. There are no data on the optimal treatment of such patients who are ineligible for ASCT and would not be expected to tolerate such regimens well. Numerous trials of first-line treatment, especially for early stage disease, demonstrate the superior disease control achieved with combined modality therapy. It therefore seems reasonable to combine radiotherapy (RT) with chemotherapy for transplant-ineligible patients at relapse. This would be particularly attractive for those patients with limited stage disease and for those who have either not received radiotherapy as part of first-line treatment or who have relapsed outside of the previous radiation field. In patients unlikely to tolerate the toxicities associated with more intensive regimens, limited published experience with single agent palliative strategies, such as vinblastine, lomustine, etoposide or gemcitabine (Mead et al, 1982; Haim et al, 1995; Little et al, 1998; Zinzani et al, 2000), or multi-agent oral regimens with or without intravenous vinblastine, such as PECC (prednisolone, etoposide, CCNU [lomustine], chlorambucil)(Proctor et al, 2010) or ChlVPP (chlorambucil, vinblastine, procarbazine, prednisolone)(Selby et al, 1990), suggest therapeutic benefits may be achieved in many cases. The role of newer agents, such as brentuximab vedotin, in this setting requires further evaluation. The early input of palliative care specialists is recommended.
Recommendations
- The choice of a first line salvage regimen in patients eligible for ASCT should be based on patient factors and familiarity of the treatment centre with the regimen (2C) (Fig 1).
- Regimens containing stem cell toxic agents (such as carmustine and melphalan) should be avoided if possible until stem cells have been successfully collected and cryopreserved if ASCT is planned (1B).
- There is currently no evidence to support intensive sequential induction/consolidation strategies prior to ASCT (1B).
- Consider switching to an alternative non-cross-resistant salvage regimen if there are residual FDG-avid lesions after first line salvage treatment and the intent is to proceed to ASCT (2B).
- In patients not eligible for ASCT, combined modality therapy should be considered, especially in early stage relapse and in patients who have not received prior radiotherapy or who have relapsed outside of the initial radiotherapy field (2B).
- In patients unlikely to tolerate the toxicities associated with more intensive regimens, palliation with either a single agent or with a multi-agent oral regimen with or without intravenous vinblastine should be considered (2C).
- Early consideration of involvement of palliative care services is recommended, particularly in those not eligible for high dose therapy (1C).
6. Autologous stem cell transplantation
The outcome of patients with relapsed or refractory disease treated with conventional doses of chemotherapy alone is generally poor with durable remission rates of between 10 and 35% (Longo et al, 1992; Linch et al, 1993; Schmitz et al, 2002), the higher rates often reflecting long term outcomes only in the subset achieving at least a PR to initial salvage therapy. Patients relapsing several years after initial treatment may do better with conventional salvage chemotherapy alone, but long term PFS remains below 50% (Yuen et al, 1997). Although no overall survival (OS) benefit has ever been demonstrated in a prospective, randomized clinical trial, the aim of treatment at relapse in younger patients without significant co-morbidities is to induce remission and then proceed to high dose therapy with ASCT. This recommendation is based on two randomized trials, which demonstrated a significant benefit of ASCT over conventional chemotherapy in terms of FFTF for patients with relapsed disease (Linch et al, 1993; Schmitz et al, 2002). The lack of a survival benefit in either of these two studies has been attributed to patients in the non-ASCT arm undergoing transplant at the time of second relapse. Both trials used the BEAM conditioning regimen prior to ASCT. In the first, this was compared to 1–3 cycles of mini-BEAM (Linch et al, 1993), and in the second compared to two cycles of Dexa-BEAM following initial induction with two cycles of Dexa-BEAM (Schmitz et al, 2002). Both trials determined response to salvage therapy according to conventional CT criteria, excluding those with less than PR from subsequent randomization. A number of other single-arm institutional and registry series confirm similar outcomes following ASCT. BEAM remains the most popular regimen worldwide, but other conditioning regimens with comparable toxicities and outcomes have been reported in single-institution studies (Moskowitz et al, 2001; Lavoie et al, 2005; Wadehra et al, 2006; Perz et al, 2007; Benekli et al, 2008; Visani et al, 2011; Nieto et al, 2013). Total body irradiation (TBI)-based regimens have been largely abandoned in favour of chemotherapy-based regimens because of a higher incidence of secondary malignancies and transplant-related mortality with the former (Sureda et al, 2001). Much like the experience with salvage chemotherapy (see below), there are no prospective data to suggest the superiority of one conditioning regimen to another, and the choice of conditioning regimen is therefore usually based on institutional preference and experience.
Relapses occurring more than 5 years after primary therapy are rare, but occur at a higher incidence than the incidence of HL in the general population, suggesting that this does represent a relapse of the original disease (Gaudio et al, 2011). Although data are scarce, outcomes appear favourable in terms of response rates using either a second course of the primary treatment regimen or an alternative non-cross-resistant regimen. Toxicities and mortality associated with treatment complications are relatively high (Provencio et al, 2010; Gaudio et al, 2011), and the majority of reported cases did not undergo ASCT as consolidation. At present there is insufficient data to recommend routine ASCT in those achieving a complete metabolic response, although it is a reasonable clinical option.
6.1 Post-ASCT maintenance
As in other clinical settings, cytotoxic agents have been investigated as possible post-ASCT maintenance therapies, but such strategies have met with limited success. For example, an attempt to consolidate ASCT with involved field radiotherapy (IFRT) to sites of pre-existing disease of >2 cm, followed by two cycles each of alternating DCEP-G, (dexamethasone, cyclophosphamide, etoposide, cisplatin, gemcitabine), and DPP (dexamethasone, cisplatin, paclitaxel), administered every 3 months until 1 year post-transplant, was notable for the fact that only 17/37 (46%) received the planned post-ASCT therapy, either because of refusal, early relapse or other complications (Rapoport et al, 2004). Despite the inclusion of 25 (68%) patients with relapsed rather than refractory disease, and 33 (89%) with only 1–2 lines of prior therapy, the PFS at 2·5 years was only 59%. Further investigation of agents that are potentially less toxic when administered as maintenance (e.g., brentuximab vedotin or panobinostat) are eagerly awaited, but current evidence does not support maintenance strategies.
6.2 Tandem ASCT
A potential role for tandem ASCT has been explored in a number of non-randomized studies. The H96 trial used a risk-adapted approach, reserving tandem ASCT for patients with two or more of three adverse risk factors, which included relapse or progression <12 months, stage III-IV disease at relapse, and relapse in a previously irradiated site (Morschhauser et al, 2008). The first conditioning regimen consisted of cyclophosphamide, carmustine, etoposide, and mitoxantrone (CBVMx) and the second of TBI (or busulphan for patients who had received prior dose-limiting radiation) and melphalan. Their outcomes were compared to those of low-risk patients who underwent a single BEAM-conditioned ASCT. The outcomes for the poor-risk patients remained inferior to those of the intermediate-risk patients, with 5-year FF2F rates of 46% and 73%, although it was suggested that results might have been superior to those of similarly high-risk cohorts in earlier series using single ASCT. Confirmation will require a prospective randomized study, and tandem ASCT cannot currently be recommended outside of clinical trials.
6.3 Relapse post ASCT
Outcomes for patients who relapse following ASCT, particularly for those with early relapse within 6–12 months, have historically been poor. Even in more recent series, the median OS has been only 25–32 months (Moskowitz et al, 2009; Kaloyannidis et al, 2012). The aim of treatment in these patients is to attain sufficient response to allow consideration of allogeneic transplantation in those deemed eligible (see section 5·1). In those not deemed appropriate candidates for allogeneic transplantation, therapy should be individualized according to specific circumstance. Some patients will be most appropriately treated with a palliative approach, and early involvement of specialist palliative services is recommended. In the majority, further attempts to gain disease control are warranted, recognizing that some will achieve prolonged periods of disease control, particularly those relapsing later following ASCT (Martinez et al, 2013). Brentuximab vedotin should be considered amongst the alternative regimens at this stage, although choice may be modulated by prior history of exposure. Although the median OS in the pivotal study was quoted as 22·4 months, response rates were high and toxicities modest (Younes et al, 2012).
Recommendations
- ASCT is the standard treatment for patients with relapsed disease who achieve an adequate response to salvage therapy (1A) (Fig 1).
- ASCT is also the standard treatment for patients with primary resistant disease who achieve an adequate response to salvage therapy (1B).
- ASCT is not recommended in those failing to achieve an adequate response (1B).
- An adequate response to salvage therapy is currently defined as a PR by conventional CT criteria (2B).
- Choice of conditioning regimen should be based on familiarity of the treatment centre with the regimen (2C).
- Current evidence does not support the use of maintenance cytotoxic therapies post-ASCT (1C).
- Tandem ASCT cannot currently be recommended outside of clinical trials (1C).
7. Allogeneic haematopoietic stem cell transplantation (HSCT)
The role of allogeneic HSCT in the management of this patient group remains controversial. Whilst application has historically been limited by prohibitive procedure-related mortality rates, improvements in supportive care coupled to the introduction of ‘reduced toxicity’ conditioning strategies has allowed re-evaluation of a possible role. The inverse correlation between relapse and the development of graft-versus-host disease following allogeneic HSCT, along with responses to donor lymphocyte infusions, confirms the existence of a therapeutically relevant graft-versus-lymphoma activity (Peggs et al, 2005, 2007, 2011; Devetten et al, 2009; Robinson et al, 2009; Sureda et al, 2012). Currently, there are no comparative data to definitively direct the choice of preparative regimen. Nevertheless, a strategy employing a single, more intensive preparative regimen may be preferable to a tandem ASCT-allogeneic HSCT strategy, based on economic considerations and overall duration of treatment.
7.1 For patients relapsing after ASCT
The majority of early experience was in those with relapse following ASCT, and these patients still probably represent the majority of those proceeding to allogeneic HSCT. Current data suggest that the reduced non-relapse-related mortality associated with reduced intensity conditioning is, to some degree, offset by increased relapse rates (Sureda et al, 2008; Claviez et al, 2009; Devetten et al, 2009). PFS rates in this setting range from 20 to 40% at 2–4 years in larger series and registry datasets (Anderlini et al, 2008; Sureda et al, 2008, 2012; Peggs et al, 2011). Appropriately human leucocyte antigen (HLA)-matched unrelated donors yield comparable outcomes to those achieved with HLA-matched related donors in most series (Robinson et al, 2009; Peggs et al, 2011; Sureda et al, 2012). Most studies, though not all (Devetten et al, 2009), identify chemo-sensitivity at the time of transplant as an important prognostic indicator (Robinson et al, 2009), although definitions for resistant disease vary. Those with progressive disease have almost uniformly poor outcomes and cannot be recommended for transplant (Robinson et al, 2009; Sureda et al, 2012). The situation is less clear for those with disease that remains stable following salvage (Peggs et al, 2005; Sureda et al, 2012). For those with chemo-sensitive disease, allogeneic HSCT may be the most attractive clinical option, offering the possibility of prolonged disease-free survival (DFS) and potentially cure to a sizeable minority. Two retrospective analyses of patients who relapsed after an ASCT suggest that, for those patients with a HLA-compatible donor and who responded sufficiently to salvage to enable allogeneic HSCT to occur (Thomson et al, 2008; Sarina et al, 2010), consolidation with a reduced-intensity transplant offers a better long-term outcome than the use of conventional strategies, with a significant advantage (P < 0·001) in a donor versus no donor comparison of 185 patients for both OS and PFS. A further retrospective study on behalf of EBMT/Gruppo Italiano Trapianto Di Midollo Osseo (GITMO) that included 244 patients who had relapsed after an ASCT showed a trend in the same direction for improved OS (P = 0·08) in those undergoing allogeneic HSCT (Martinez et al, 2013). For patients relapsing very late after ASCT, a second ASCT may be a reasonable therapeutic option (Thomson et al, 2007). No prospective comparative studies exist to inform recommendations regarding the most appropriate salvage regimen(s) post-ASCT. In general, the use of an agent(s) that the patient has not been exposed to previously is suggested. Brentuximab vedotin clearly has impressive single-agent activity in the setting of relapse post ASCT, although the majority of patients fail to achieve CR, and the time-to-progression in these cases is short (<5 months). In these cases brentuximab vedotin may offer a useful bridge to allogeneic transplant, with significant benefits in terms of its toxicity profile (Chen et al, 2012). For patients achieving CR following brentuximab vedotin the situation is less clear, though these patients proabably have the best outcomes following allogeneic HSCT, and experience with other salvage regimens implies that transplantation at the time of maximal disease control is preferable.
7.2 For patients failing to achieve metabolic complete response
The more recent investigation of a response-adjusted transplantation algorithm identifies a further potential strategy for evaluation of allogeneic HSCT in those deemed to be at high risk of failure of ASCT, targeting the intensification to those who have residual FDG-avid disease following salvage therapy (Thomson et al, 2013). The 3-year PFS of 68% in this high-risk group was encouraging, with 80% ‘current’ PFS following donor-lymphocytes. Such approaches may require refinement according to delineation of number of lines of salvage, and according to the outcome of prospective studies evaluating maintenance strategies following ASCT (e.g. the AETHERA trial), and it is recommended that they be evaluated within the context of prospective national studies.
Recommendations
- Allogeneic transplantation using a reduced intensity conditioning regimen is the treatment of choice for younger patients with a suitable donor and chemo-sensitive disease following failure of ASCT (2B) (Fig 1).
- An appropriately HLA-matched unrelated donor should be considered when there is no HLA-matched sibling (2B).
- A second autologous transplant is a reasonable clinical option in selected patients with late relapse following ASCT (2C).
- Investigation of the use of allogeneic transplantation earlier in the treatment pathway should be performed in the context of prospective clinical trials, but may be justified in selected patients who have required multiple lines of therapy to achieve a response (2C).
8. Radiotherapy
The propensity of HL to relapse and progress in previously involved sites (Mundt et al, 1995) and the radiosensitivity of HL makes RT a potentially important treatment modality in this setting. The move towards less extensive use of RT in primary therapy also raises the possibility of a greater role in relapsed or refractory patients. RT is well documented to enhance local disease control in sites of refractory or recurrent HL (Vose et al, 1992; Wirth et al, 1997; Josting et al, 2005). Radiation treatment volumes are localized to encompass the known site(s) of disease recurrence, without prophylactic inclusion of adjacent lymph nodal stations. Overall, salvage RT is safe and well tolerated with mild to moderate acute reversible side effects including fatigue, anorexia, nausea, skin erythema, and dysphagia. The risks of late toxicity should always be balanced against the risks associated with disease progression in this relapsed and refractory setting by experts in radiotherapy of HL, working within the multidisciplinary team. The risk of late toxicity and second cancer risk is dependent on the site, volume and type of tissue irradiated, as well as age and sex of the patient (Hodgson, 2011) and references therein).
8.1 Salvage radiotherapy
Salvage RT plays an important role in local control for patients who have primary refractory disease dominated by a local site, as well as those who relapse after initial therapy, where RT is generally used as part of combined modality therapy along with salvage chemotherapy, prior to ASCT. A small group of patients with localized disease and no systemic symptoms enjoy prolonged DFS with RT alone (Josting et al, 2005). RT should also be considered as a salvage option in the setting of ASCT failure, after relapse or progression, where a significant proportion of patients still achieve high response rates to salvage RT and a few may even enjoy long term DFS of over 5 years, whilst in others it plays an important role in palliation (Goda et al, 2012). Systemic failures remain the commonest problem in this setting, underlining the need for improved systemic therapy in combination with salvage RT.
8.2 Radiotherapy in conjunction with ASCT
RT has been used for cytoreduction and consolidation therapy in the peri-transplant period in some transplant programmes world wide (Moskowitz et al, 2001), and has been reported to result in low numbers of ASCT failures in patients who received RT in single institution studies. It should be considered in patients that have a dominant site of local relapse at an initially involved site (these are usually patients who have had bulky disease with residual abnormalities following salvage chemotherapy and ASCT) (Poen et al, 1996; Wendland et al, 2006; Biswas et al, 2012). For peri-transplant RT, the radiation volumes are constructed using international guidelines (Specht et al, 2013) and are determined on an individual patient basis depending on the sites of disease at initial diagnosis and at relapse. Patients who are candidates for salvage therapy may benefit from RT either before or after ASCT to sites of dominant local recurrence. In patients with complete response to salvage chemotherapy, a dose of 30–36 Gy post ASCT is recommended (Specht et al, 2013). In addition, RT should be delivered as soon as the patient has recovered from the acute side effects of ASCT, and ideally within 6 weeks following stem cell infusion. Consideration should be given to previous RT and to the radiosensitivity of normal tissues and organs that would be inadvertently irradiated.
Recommendations
- The use of radiotherapy should be given serious consideration in cases of local relapse or relapse at sites where local disease is dominating the clinical picture. The use of involved site techniques is recommended to minimize toxicity to normal tissues (for example, lung fields) if subsequent high dose consolidation therapy is planned (2B) (Fig 1).
- Salvage radiotherapy alone may be considered a reasonable treatment option in selected patients not eligible for ASCT, especially for older patients with relapsed HL who lack B symptoms, have a good performance status, and have limited stage disease at relapse (2B).
- In the rare event of late relapse >5 years after primary therapy occurring at a localized site without B symptoms, treatment with standard-dose chemotherapy and involved field radiation alone may be appropriate (2B).
- Peri-transplant (ASCT) radiotherapy should be considered in patients that have a dominant site of local relapse at an initially involved site (these are usually patients who have had bulky disease with residual abnormalities following salvage chemotherapy and ASCT) (2C).
Summary of key recommendations
- Repeat biopsy is generally recommended in Hodgkin lymphoma (HL) patients thought to have relapsed, and should be considered in those who have residual fluorodeoxyglucose (FDG)-avid lesions post-therapy (1C).
- Positron-emission tomography/computerized tomography (PET-CT) is the preferred restaging modality after salvage therapy (1B).
- The aim of salvage treatment should be to achieve an FDG-PET-negative remission (1B).
- The choice of a first-line salvage regimen in patients eligible for autologous stem cell transplantation (ASCT) should be based on patient factors and familiarity of the treatment centre with the regimen (2C).
- Regimens containing stem cell toxic agents (such as carmustine and melphalan) should be avoided if possible until stem cells have been successfully collected and cryopreserved if ASCT is planned (1B).
- There is currently no evidence to support intensive sequential induction/consolidation strategies prior to ASCT (1B).
- Consider switching to an alternative non-cross-resistant salvage regimen if there are residual FDG-avid lesions after first line salvage treatment and the intent is to proceed to ASCT (2B).
- In patients not eligible for ASCT, combined modality therapy should be considered especially in early stage relapse and in patients who have not received prior radiotherapy or who have relapsed outside of the initial radiotherapy field (2B).
- In patients unlikely to tolerate the toxicities associated with more intensive regimens, palliation with either a single agent or with a multi-agent oral regimen with or without intravenous vinblastine should be considered (2C).
- Early consideration of involvement of palliative care services is recommended, particularly in those not eligible for high dose therapy (1C).
- ASCT is the standard treatment for patients with relapsed disease who achieve an adequate response to salvage therapy (1A).
- ASCT is also the standard treatment for patients with primary resistant disease who achieve an adequate response to salvage therapy (1B).
- ASCT is not recommended in those failing to achieve an adequate response (1B).
- An adequate response to salvage therapy is currently defined as a partial response by conventional CT criteria (2B).
- Choice of conditioning regimen should be based on familiarity of the treatment centre with the regimen (2C).
- Current evidence does not support the use of maintenance cytotoxic therapies post-ASCT (1C).
- Tandem ASCT cannot currently be recommended outside of clinical trials (1C).
- Allogeneic transplantation using a reduced intensity conditioning regimen is the treatment of choice for younger patients with a suitable donor and chemo-sensitive disease following failure of ASCT (2B).
- An appropriately human leucocyte antigen (HLA)-matched unrelated donor should be considered when there is no HLA-matched sibling (2B).
- A second autologous transplant is a reasonable clinical option in selected patients with late relapse following ASCT (2C).
- Investigation of the use of allogeneic transplantation earlier in the treatment pathway should be performed in the context of prospective clinical trials, but may be justified in selected patients who have required multiple lines of therapy to achieve a response (2C).
- The use of radiotherapy should be given serious consideration in cases of local relapse or relapse at sites where local disease is dominating the clinical picture. The use of involved site techniques is recommended to minimize toxicity to normal tissues (for example, lung fields) if subsequent high dose consolidation therapy is planned (2B).
- Salvage radiotherapy alone may be considered a reasonable treatment option in selected patients not eligible for ASCT, especially for older patients with relapsed HL who lack B symptoms, have a good performance status, and have limited stage disease at relapse (2B).
- In the rare event of late relapse >5 years after primary therapy occurring at a localized site without B symptoms, treatment with standard-dose chemotherapy and involved field radiation alone may be appropriate (2B).
- Peri-transplant (ASCT) radiotherapy should be considered in patients that have a dominant site of local relapse at an initially involved site (these are usually patients who have had bulky disease with residual abnormalities following salvage chemotherapy and ASCT) (2C).
Disclaimer
While the advice and information in these guidelines is believed to be true and accurate at the time of going to press, neither the authors, the British Society for Haematology, nor the publishers accept any legal responsibility for the content of these guidelines.
Disclosures
GP Collins, T Illidge, A Sureda, DC Linch, and KS Peggs have acted as consultants for and/or received speaker fees from Takeda. There are no other relevant conflicts of interest to declare.
Acknowledgements
All authors contributed to writing the paper; KS Peggs edited the final manuscript. KS Peggs, K Ardeshna and DC Linch receive funding from the Department of Health's NIHR Biomedical Research Centres funding scheme.
Appendix 1
Strength of recommendations
Strong (grade 1): Strong recommendations (grade 1) are made when there is confidence that the benefits do or do not outweigh harm and burden. Grade 1 recommendations can be applied uniformly to most patients. Regard as ‘recommend’.
Weak (grade 2): Where the magnitude of benefit or not is less certain a weaker grade 2 recommendation is made. Grade 2 recommendations require judicious application to individual patients. Regard as ‘suggest’.
Quality of evidence
- (A) High. Further research is very unlikely to change confidence in the estimate of effect. Current evidence derived from randomized clinical trials without important limitations.
- (B) Moderate. Further research may well have an important impact on confidence in the estimate of effect and may change the estimate. Current evidence derived from randomized clinical trials with important limitations (e.g. inconsistent results, imprecision - wide confidence intervals or methodological flaws - e.g. lack of blinding, large losses to follow up, failure to adhere to intention to treat analysis), or very strong evidence from observational studies or case series (e.g. large or very large and consistent estimates of the magnitude of a treatment effect or demonstration of a dose-response gradient).
- (C) Low. Further research is likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. Current evidence from observational studies, case series or just opinion.




