Mutations of the GATA2 and CEBPA genes in paediatric acute myeloid leukaemia
Hereditary GATA2 mutations show predisposition to acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS) (Hahn et al, 2011). These mutations have also been reported in chronic myeloid leukaemia (Zhang et al, 2008) and monocytopenia and mycobacterial infection (MonoMAC) syndrome (Hsu et al, 2011). More recently, GATA2 mutations have been identified in de novo AML, especially in adult patients with biallelic CEBPA mutations (Greif et al, 2012; Green et al, 2013). GATA2 and CEBPA are transcription factors that are crucial for haematopoietic development. These findings prompted us to identify possible GATA2 and CEBPA mutations in patients with various paediatric leukaemias.
Direct Sequencing of GATA2 was performed in 157 de novo AML patients, including 13 patients with acute promyelocytic leukaemia (APL; French–American–British type-M3) and 10 with Down syndrome (DS; Table S1), 22 secondary AML patients, 40 juvenile myelomonocytic leukaemia (JMML) patients, 50 acute lymphoblastic leukaemia (ALL) patients, 70 cell lines (25 B-cell precursor-ALL, 15 T-cell-ALL, 22 AML, and 8 neuroblastomas), and 60 healthy subjects. GATA2 mutation analysis was performed by direct sequencing for all coding exons (exons 2–6) using an ABI PRISM 3130 Genetic Analyser (Applied Biosystems, Branchburg, NJ, USA) (Table S2). For AML patients, CEBPA and NPM1 mutations were also examined. Mutational analyses of FLT3, KIT, WT1 and RAS genes in our AML patients was performed as described previously (Shimada et al, 2006). Informed consent was obtained from the patients or the patients' parents according to guidelines based on the tenets of the revised Helsinki protocol. The institutional review boards of Gunma Children's Medical Centre approved this project.
GATA2 mutations were found in eight out of 157 AML patients (5·1%), including three APL patients (Fig 1A,B), but were absent in 18 patients with acute megakaryocytic leukaemia (FAB-M7; Table S3). Furthermore, there were no GATA2 mutations in patients with other leukaemias, in the cell lines, or in the 60 healthy subjects, suggesting that GATA2 mutations were indeed associated with leukaemogenesis in a subset of patients with de novo AML.
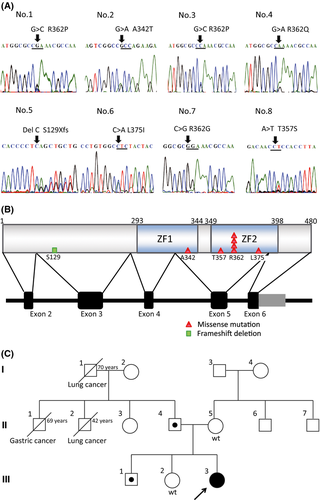
Germline GATA2 mutations were also examined in five AML patients whose complete remission (CR) samples were available, and a germline mutation was identified in one patient. Furthermore, we performed GATA2 mutation analyses of the patient's parents and two siblings, and identified the same GATA2 mutations in her father (II-4) and brother (III-1) but not in her mother (II-5) or sister (III-2) (Fig 1C). Her father and brother lacked abnormalities in their full blood cell counts, lymphocyte subsets, or episodes of opportunistic infections. The proband experienced severe mycotic pneumonia during induction chemotherapy. Remarkably, she has been in CR for more than 11 years, despite discontinuation of chemotherapy. Three patients, for whom CR samples were not available, had no history of MonoMAC syndrome.
In addition, 16 CEBPA mutations (10·2%) and three NPM1 mutations (1·9%) were found in 157 paediatric AML patients. Thirteen (81·3%) of 16 patients with CEBPA mutations had been in CR for more than 4 years, suggesting that CEBPA mutations may be associated with favourable outcomes. Although most GATA2 mutations were found in patients with biallelic CEBPA mutations in adult AML (Greif et al, 2012; Green et al, 2013), only two of eight GATA2 mutation-positive patients had monoallelic CEBPA mutations in this study (Table 1).
| Pt | Sex | Age (years) | FAB | WBC (× 109/l) | Chromosome | Risk | Tx | Relapse | Prognosis (months) | GATA2 mutation | Germline | Additional mutations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 3 | M4 | 23·8 | 46, XY, t(11;19) (q23; p13·1) | IR | Auto | Yes | 16 | R362P | N/A | – |
| 2 | F | 7 | M0 | 3·7 | 45,XX,add(3)(p13),del(6)(q?), der(8) t(3;8)(p21;q24), −13 | IR | Chemo | No | +141 | A342T | Yes | NRAS |
| 3 | F | 8 | M1 | 1·8 | 46, XX | IR | Chemo | No | +56 | R362P | No | KRAS |
| 4 | M | 14 | M1 | 440·0 | 46XY [2/8], 46, XY, del(6) (q15 q21), −7, −9, −10, +3mar[1/8], 46, XY, ?de(3) (p25)[1/8], 47, XY, −5, −8, −10, add(12)(q24·1), −16, −18, +6mar [1/8], 46, XY, −2, −6, −8, +3mer [1/8], 46, XY, −8, +mar [1/8], 46, Y, ?add(X)(p11·2) [1/8] | IR | Auto | No | +51 | R362Q | No | WT1, CEBPA-SM |
| 5 | M | 11 | M3 | 16·1 | 46,XX,inv(9)(p11q13),t(15;17)(q22;q11-21) | M3 | Chemo | No | +50 | S129X | N/A | – |
| 6 | M | 3 | M3 | 11·6 | 46,XY,t(15;17)(q22;q11?21) | M3 | Chemo | No | +45 | L375I | No | CEBPA-SM |
| 7 | M | 10 | M3 | 13·6 | 47,XY, +8, t(15;17)(q22;q11-21) | M3 | Chemo | No | +41 | R362G | N/A | KIT |
| 8 | F | 2 | M4 | 12·7 | 48, XX, +6, +10, t(11?; 7) (q23;q25) | IR | Chemo | No | +38 | T357S | No | – |
- Pt, Patient; FAB, French–American–British classification; WBC, white blood cell count; Tx, Treatment; M, Male; F, Female; IR, Intermediate risk; Auto, Autologous stem cell transplantation; Chemo, Chemotherapy; N/A, not available; +, alive; SM, single mutation.
We compared the clinical and molecular features between patients with and without GATA2 mutations. However, there were no significant differences in terms of age, initial white blood cell count, gender, and cytogenetics (Table S3). Of the eight patients with GATA2 mutations, one had a WT1 mutation, one had a KIT mutation, and two patients had RAS mutations (Table 1). FLT3-internal tandem duplication, MLL-partial tandem duplication, and NPM1 mutations were not found in any patients with GATA2 mutations (Table S3). All of the GATA2 mutations were found in the intermediate risk subgroup or APL patients with t(15;17), whereas none were found in those with core-binding factor AML [i.e. t(8;21) and inv(16)]. GATA2 mutations were found in two patients with 11q23 translocations, including t(11;19) and t(7;11), and three patients with complex chromosomal abnormalities, whereas most GATA2 mutations were found in cytogenetically normal AML patients in previous reports (Table 1) (Greif et al, 2012; Luesink et al, 2012).
GATA2 mutations were previously reported in patients with M1, M2, and M4 subtypes of AML (Greif et al, 2012; Luesink et al, 2012), which is in accordance with our results. GATA2 mutations have not been previously reported in APL, but our study found these mutations in three APL patients. Of note, promyelocytic leukaemia protein has been shown to interact with GATA2 and potentiate its transactivation capacity (Tsuzuki et al, 2000).
The outcomes of our patients with GATA2 mutations was not poor (3-year overall survival and event free survival: 87·5%), which is in agreement with previous reports on de novo AML (Greif et al, 2012; Luesink et al, 2012): two of eight patients received autologous-stem cell transplantation (Auto-SCT), and one died of gastrointestinal haemorrhage after Auto-SCT. The remaining six patients who did not receive Auto-SCT were still alive (Table 1).
In this study, one patient with a germline GATA2 mutation developed AML. Her paternal grandfather (I-1) and second uncle (II-2) died of lung cancer at the age of 70 and 42 years, respectively, while her first uncle (II-1) died of gastric cancer at 69 years of age (Fig 1C). Increased GATA2 protein expression has been associated with biochemical recurrence and distant metastatic progression in prostate cancer (Böhm et al, 2009), as loss of GATA2 reduced the viability of Non-small cell lung cancer cells with RAS-pathway mutations, whereas wild-type cells were unaffected (Kumar et al, 2012). These facts indicate that GATA2 upregulation is strongly associated with maintenance of cancer cells. The association between GATA2 mutations and solid tumours remains to be elucidated.
Our results indicate that GATA2 mutations are associated with a favourable outcome in paediatric AML. Therefore, less aggressive treatment strategies without SCT may be appropriate for paediatric AML patients with GATA2 mutations, although most patients with GATA2 mutations were classified into an intermediate risk group. Furthermore, the association between germline GATA2 mutations and solid tumours remains to be elucidated.
Acknowledgements
We are grateful to all members of the Japanese Childhood AML Cooperative Study Group. Members of the Japanese Childhood AML Cooperative Study Group who contributed data to the study include Ryoji Hanada, Department of Haematology/Oncology, Saitama Children's Medical Centre; Masahiro Tsuchida, Department of Haematology/Oncology, Ibaraki Children's Medical Centre; Akira Morimoto, Department of Paediatrics, Kyoto Prefectural University of Medicine; Ryoji Kobayashi, Department of Paediatrics, Hokkaido University School of Medicine; Hiromasa Yabe, Department of Paediatrics, Tokai University School of Medicine; Kazuko Hamamoto, Department of Paediatrics, Hiroshima Red Cross Hospital; Shigeru Tsuchiya, Department of Paediatric Oncology, Institute of Development, Aging and Cancer, Tohoku University; Yuichi Akiyama, Department of Paediatrics, National Hospital Organization Kyoto Medical Centre; Hisato Kigasawa, Department of Haematology, Kanagawa Children's Medical Centre; Akira Ohara, Department of First Paediatrics, Toho University School of Medicine; Hideki Nakayama, Department of Paediatrics, Hamanomachi Hospital; Kazuko Kudo, Department of Paediatrics, Nagoya University Graduate School of Medicine; and Masue Imaizumi, Department of Haematology/Oncology, Miyagi Prefectural Children's Hospital. The authors would like to thank Enago (www.enago.jp) for the English language review. This work was supported by a grant for Cancer Research, a grant for Research on Children and Families, and Research on Intractable Diseases, Health and Labour Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan, by Grants-in-Aid for Scientific Research (B, C) and Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the Programme for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NiBio) of Japan, and by a Research grant for Gunma Prefectural Hospitals.
Author contributions
Y.H. designed the study; M.F., S.A., M.K., A.K., M.S., A.T., K.H. and I.T. collected patient samples and clinical data; N.S., K.O., M.-J.P., Y.M. and S.M. performed the laboratory research; N.S., M.-J.P. and Y.H. analysed and interpreted the data; N.S. performed the statistical analysis; N.S. and Y.H. wrote the manuscript; H.A. and Y.H. supervised the work; and all authors critically reviewed the manuscript and gave their final approval.
Conflicts of interest
The authors declare no competing financial interests.




