Implication of MAPK1/MAPK3 signalling pathway in t(8;9)(p22;24)/PCM1-JAK2 myelodysplastic/myeloproliferative neoplasms
Translocations involving the JAK2 ‘Janus-activated kinase 2’ gene have been described in both lymphoid and myeloid haematological malignancies. Among them, t(8;9) leading to PCM1 ‘pericentriolar material 1’-JAK2 fusion events are extremely rare with <30 reported clinical cases (Bousquet et al, 2005; Heiss et al, 2005; Murati et al, 2005 Reiter et al, 2005; Patnaik et al, 2010; Dargent et al, 2011; Lierman et al, 2012). Although the clinical onset of these disorders is widely heterogeneous, most of them present with a myelodysplastic/myeloproliferative disease with striking dysplastic features of the erythroid compartment (Heiss et al, 2005; Dargent et al, 2011).
Given that allogenic stem cell transplant is the only curative option for eligible patients, several therapeutic approaches (interferon-α, hydroxycarbamide, conventional chemotherapy) have been employed to act as a bridge to transplantation or for use in unfit patients (Murati et al, 2005).
Very recently, Lierman et al (2012) showed that ruxolitinb, a JAK1/2-inhibitor approved for high-risk myelofibrosis, could inhibit in-vitro growth and JAK2/STAT5 phosphorylation of the PCM1-JAK2-transformed Ba/F3 murine cell line.
However, the status of JAK/STAT signalling has not been assessed in primary cells from PCM1-JAK2 patients.
Here, for the first time, we characterize: (i) the erythroid differentiation capacity of ex-vivo expanded CD34+ cells, and (ii) the signalling pathways activated in circulating neoplastic cells (CNCs) from a patient diagnosed with atypical chronic myeloid leukaemia carrying t(8;9)(p22;p24)/PCM1-JAK2.
A 29-year-old Caucasian male presented with the typical stigmata of a chronic myeloproliferative disorder: leucocytosis (67·1 × 109/l) with circulating myeloid progenitors, mild anaemia (124 g/l), thrombocytopenia (130 × 109/l) and splenomegaly. Bone marrow (BM) histology was hypercellular. The most prominent feature was represented by a severe erythroid dyplasia, showing abundant large peritrabecular clusters of proerythroblasts with marked reduction of the mature erythroid compartment (Fig 1A,B).

Cytogenetics showed 46,XY,t(8;9)(p22;p24) in 87% of metaphases and follow-up break-apart FISH assays for JAK2/9p24, RP11-125K10 (5′JAK2) and RP11-39K24 (3′JAK2) and for PCM1/8p22, RP11-156K13 (5′PCM1) and RP11-484L21 (3′PCM1) confirmed the PCM1-JAK2 rearrangement (Fig 1C).
Chimeric PCM1-JAK2 fusion transcript was also detected in CNCs by nested reverse transcription polymerase chain reaction (RT-PCR) (Fig 1D, lane 1) performed as previously described (Reiter et al, 2005). Specificity of the assay was confirmed by the absence of PCM1-JAK2 transcript signal in a healthy subject (HS) (Fig 1D, lane 8).
The patient underwent two courses of acute myeloid leukaemia-like chemotherapy followed by allogenic bone marrow transplant from a human leucocyte antigen (HLA)-identical sibling donor. While no molecular response was achieved with the chemotherapy only (Fig 1D, lanes 2–5), no pathological transcript was detectable by nested RT-PCR in both bone marrow mononuclear cells and peripheral blood mononuclear cells (PBMCs) at day 100 post-transplantation (Fig 1D, lanes 6–7).
Haematopoietic progenitors taken from polycythaemia vera (PV) patients that bear the constitutively-active JAK2 V617F mutation display cytokine hypersensitivity (Ugo et al, 2004).
To test whether this was the case in our patient, we isolated primary CD34+ cells from our PCM1-JAK2 fusion patient, a JAK2 V617Fpos PV and a granulocyte colony-stimulating factor-mobilized individual (M) using the CD34+ cell isolation kit (Miltenyi Biotech, Gladbach, Germany).
CD34+ cells were plated at a cell density of 1 × 106 cells/ml and then cultured for 14 d in serum-free X-vivo medium supplemented with 3 ng/ml recombinant human interleukin-3 (rIL3), 50 ng/ml stem cell factor (SCF, also known as KITLG) and 5 U/ml erythropoietin (EPO). At days 9, 11 and 14 of culture, cell number and viability was determined by trypan-blue staining. On the 14th day of culture, we stained cells with a R-phycoerythrin (RPE)-conjugated anti-Glycophorin A (Gly-A) antibody (Dako, Glostrup, Denmark) and assessed erythroid differentiation by flow cytometry.
Surprisingly, CD34+ from the PCM1-JAK2 fusion patient displayed impaired growth capacity [fold increase (FI) = 0·6] in erythroid differentiation medium, as compared to both PV and M CD34+ cells (FI = 8 and 6·5 respectively) (Fig 2B). Additionally, CD34+ from the PCM1-JAK2 fusion patient cells showed impaired erythroid differentiation capacity, as demonstrated by the low percentage of Gly-Apos cells (3·1%) compared to both PV and M CD34+ cells (84·5% and 52·6% Gly-Apos cells, respectively) (Fig 2A). Finally, as described above, histology revealed a prominent dyserythropoietic phenotype in the bone marrow (Fig 1A,B). Collectively, these observations suggested that the ex-vivo erythroid differentiation capacity of haematopoietic progenitors from this t(8;9)(p22;p24)/PCM1-JAK2 fusion case was markedly impaired, a phenotype distinct from JAK2 V617Fpos PV (Ugo et al, 2004).
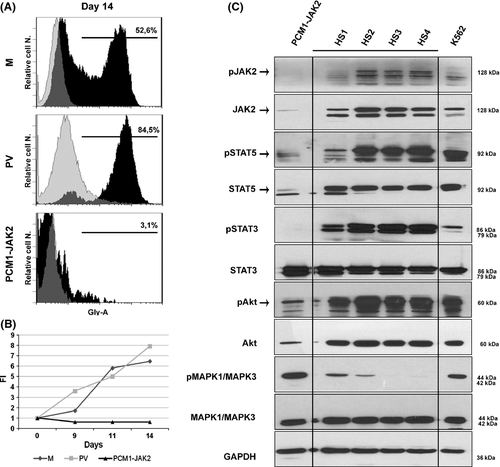
Polycythaemia vera erythroid precursors bearing the JAK2 V617F mutation often display aberrant JAK/STAT, PI3K and RAS signalling (Laubach et al, 2009). Therefore, we set out to determine the status of these pathways in CNCs harbouring the PCM1-JAK2 translocation. To this effect, protein lysates produced from PCM1-JAK2-expressing CNCs, PBMCs from four healthy subjects (HS 1–4) and K562 cells (positive control) were subjected to Western blot analyses with antibodies that separately recognize the total and phosphorylated (i.e. activated) forms of JAK2, STAT3, STAT5, Akt and mitogen-activated protein kinase 1 and 3 (MAPK1/MAPK3, also known as ERK1/2) (Cell Signaling Technology, Danvers, MA, USA).
Based on previous findings in PVs (Laubach et al, 2009), we expected to observe an activation of the above mentioned signalling pathways. However, as shown in Fig 2C, we found reduced levels of phosphorylated STAT3, total and phosphorylated JAK2, STAT5 and Akt in CNCs from our patient compared to the four healthy subjects. Furthermore, while levels of total MAPK1/MAPK3 and total STAT3 were comparable among all five conditions, CNCs derived from our patient displayed a robust increase in MAPK1/MAPK3 phosphorylation. These results indicate that the PCM1-JAK2 fusion protein is incapable of activating the JAK/STAT signalling axis but may selectively activate the RAS-MAPK pathway.
Overall, the clinical and histopathological features in this patient are indicative of a myeloproliferative stimulus associated with impaired erythroid differentiation, and the signalling findings suggest a distinct molecular mechanism of t(8;9)(p22;p24)/PCM1-JAK2 fusion neoplasms with erythroid dysplasia.
Whether PCM1-JAK2 fusion-associated malignancies arise in haematopoietic stem-cells or lineage-committed progenitors has not been determined yet. However, our data clearly show that the in-vitro behaviour of primary CD34+ cells from our patient faithfully reproduce the in-vivo findings. Additionally, the molecular dissection of the signalling cascade in our patient's CNCs is not only consistent with both the above described clinical and laboratory features, but also immediately pinpoint a potential drug-directed target in the subset of patients resistant to JAK-inhibitors.
Acknowledgements
This work was supported by: FIRB-accordo di programma quadro 2010 (IT-Ministry of the University and Scientific and Technological Research/Ministry of Education, University and Research, MIUR-RBAP10KCNS_002) to M.V.; Programma Regione-Università 2010–2012 Area 1-Regione Emilia Romagna to G.G.; AIRC-Italian Association for Research on Cancer to C.M.
Conflict of interest
The authors have no conflict of interest to disclose.




