Tofacitinib in patients with moderate-to-severe chronic plaque psoriasis: long-term safety and efficacy in an open-label extension study
Summary
Background
Tofacitinib is an oral Janus kinase inhibitor. Final safety and efficacy data from an open-label extension study of tofacitinib in psoriasis are reported.
Objectives
To evaluate the long-term safety and durability of efficacy of tofacitinib in adults with moderate-to-severe chronic plaque psoriasis.
Methods
Eligible patients who completed qualifying phase II/III tofacitinib studies received tofacitinib 10 mg twice daily (q12h) until month 3; subsequently, the dose could be adjusted by investigators to either 5 or 10 mg q12h. Adverse events (AEs) are reported up to month 66 and laboratory data up to month 54. Efficacy end points up to month 54 included Physician's Global Assessment of ‘clear’ or ‘almost clear’ (PGA response) and 75% improvement in Psoriasis Area and Severity Index (PASI 75).
Results
Overall, 2867 patients received tofacitinib, with a median treatment duration of 35·6 months. Adverse events (AEs) and serious AEs were reported in 82·5% and 13·7% of patients, respectively; 13·9% of patients discontinued owing to AEs; and 29 patients died. Incidence rates (patients with event/100 patient-years) were 1·16 for serious infections, 0·67 for malignancies and 0·26 for major adverse cardiovascular events. After initial changes in qualifying studies, most laboratory parameters were generally stable over 54 months. PGA response was achieved by 52–62% of patients and PASI 75 by 56–74% of patients at each study visit through month 54.
Conclusions
In patients with psoriasis, the safety profile of tofacitinib over 66 months was similar to previous reports in phase III studies and efficacy was sustained through 54 months (NCT01163253).
Tofacitinib is an oral Janus kinase (JAK) inhibitor. JAK inhibition by tofacitinib blocks signalling through several cytokines that are integral to lymphocyte function.1 Tofacitinib attenuates pathological immune pathways in patients with psoriasis through rapid reduction of keratinocyte JAK/signal transducer activator of transcription signalling, inhibition of the interleukin-23/T-helper 17 pathway and removal of keratinocyte-induced cytokine signalling, resulting in reduction of pathological dendritic cell and T-cell numbers to nonlesional levels.2
The efficacy and safety of tofacitinib 5 and 10 mg twice daily (q12h) in patients with moderate-to-severe plaque psoriasis has been demonstrated in phase II3 and global phase III4-7 trials of up to 56 weeks’ duration, and in this long-term extension (LTE) study based on interim data with efficacy end points reported through 24 months and safety reported over 33 months of exposure.7 The efficacy and safety of tofacitinib has also been studied in several immune-mediated inflammatory diseases, including rheumatoid arthritis;8-13 Crohn disease (NCT01470599);14, 15 psoriatic arthritis (NCT01976364);16, 17 ulcerative colitis;18, 19 and ankylosing spondylitis.20
The management of patients with moderate-to-severe plaque psoriasis often requires long-term maintenance treatment.21, 22 Here we report the final safety and efficacy data from the LTE study of tofacitinib in chronic plaque psoriasis.
Patients and methods
Patients
Full details of the inclusion and exclusion criteria for the LTE are provided in Appendix S1 (see Supporting Information). In brief, patients with moderate-to-severe chronic plaque psoriasis who were ≥ 18 years of age and had completed 12 weeks in a randomized phase II2, 3 or phase III4-7 study of tofacitinib for plaque psoriasis were enrolled. Eligibility criteria for the qualifying studies have been reported previously.2-7 Patients who participated in the phase II studies A3921047 or A3921147, or who did not enrol in the LTE within 14 days of the end-of-study visit of a qualifying phase III study, were subject to additional criteria (Appendix S1; see Supporting Information).
Study design and objectives
This was an open-label, LTE study (NCT01163253) carried out at 323 sites across 36 countries between September 2010 and June 2016 (Appendix S1; see Supporting Information). The study was terminated by the sponsor on 8 March 2016 as it had met its objectives of characterizing long-term safety and tolerability, and the last patient completed the study in June 2016. The study termination was not a result of any safety concerns.
Up to month 3 of the LTE, all patients received tofacitinib 10 mg q12h. At the month 3 visit and after, investigators could decrease the dose to 5 mg q12h, and could subsequently increase the dose back to 10 mg q12h, based on safety and efficacy considerations. Changes in dose were only permitted at scheduled study visits (every 3 months), unless a reduction to 5 mg q12h was required owing to safety concerns. The variable dose period continued until the patient discontinued study treatment or the study ended. Details of permitted concomitant topical therapies are provided in Appendix S1 (see Supporting Information).
Baseline values were those of the qualifying study for patients enrolling into the LTE within 14 days of completing qualifying study participation. For patients enrolling > 14 days after qualifying study participation, baseline values were obtained at the baseline visit of the LTE.
The study was conducted in accordance with the International Conference on Harmonisation guidelines on Good Clinical Practice and the Declaration of Helsinki, and applicable local regulatory requirements and laws. The study protocol was subject to approval by investigators’ institutional review board/independent ethics committee (IEC). All patients provided written, informed consent.
Study end points
Primary end points included the frequency and severity of adverse events (AEs), changes and abnormalities in laboratory safety data, vital signs, physical examinations, electrocardiogram (ECG) and adjudicated cardiovascular end points, and confirmed malignancies. Details of adjudication committees are included in Appendix S1 (see Supporting Information).
Secondary end points included the proportion of patients achieving a Physician's Global Assessment of ‘clear’ or ‘almost clear’ (PGA response); proportion of patients achieving ≥ 50%/≥ 75%/≥ 90% improvement from baseline in Psoriasis Area and Severity Index (PASI 50/75/90 responses, respectively); change from baseline in PASI score; patients with ≥ 125% of baseline PASI score; change from baseline in Itch Severity Item (ISI) score; change from baseline in Dermatology Life Quality Index (DLQI) score; patient-reported outcomes (PROs) of the Short Form-36 Health Survey [SF-36; physical component summary (PCS) and mental component summary (MCS) scores]; proportion of patients achieving Patient Global Assessment of ‘clear’ or ‘almost clear’ (PtGA response); and Euro-Qol 5 Dimensions Questionnaire (EQ-5D; utility score and visual analogue scale). The Psoriasis Health Care Resource Utilization Questionnaire was also administered; however, the results are not presented owing to inconsistent data collection.
Statistical analysis
The safety analysis set comprised all patients who received one or more dose of study drug. Safety data were summarized descriptively and include final data for the entire study duration through month 66 for AE reporting and through month 54 for laboratory parameters displayed by visits, owing to small patient numbers (n < 100) beyond month 54. For safety analyses, results were reported by dose groups, which were defined based on the percentage of days a patient received tofacitinib 10 mg q12h. The tofacitinib 10 mg q12h group included patients who received tofacitinib 10 mg q12h for ≥ 80% of their days in the study; the tofacitinib 5/10 mg q12h variable dosing group included patients who received tofacitinib 10 mg q12h for < 80% of their days in the study. Safety data are also reported for all patients treated with tofacitinib. Crude incidence rates (IRs) were calculated for AEs of special interest and were expressed as the number of patients with an event per 100 patient-years of exposure. IRs were obtained based on the number of patients with the event over the at-risk period from the first dose up to 28 days after the last dose of tofacitinib. The exact Poisson method was used to calculate confidence intervals (CIs) for IRs.
Secondary efficacy and PRO end point analyses were based on the full analysis set (FAS), using observed cases with no imputation for missing data. The FAS included patients who received one or more dose of study drug and excluded 12 patients from one site owing to an administrative error in obtaining appropriate IEC approval. Data are presented pooled across all tofacitinib doses; the open-label study design and permitted dose changes may confound comparison of efficacy end points between doses. Results are summarized using descriptive statistics, with no formal hypothesis testing. Similar to the laboratory parameters, secondary end point results are presented up to month 54.
Results
Patients
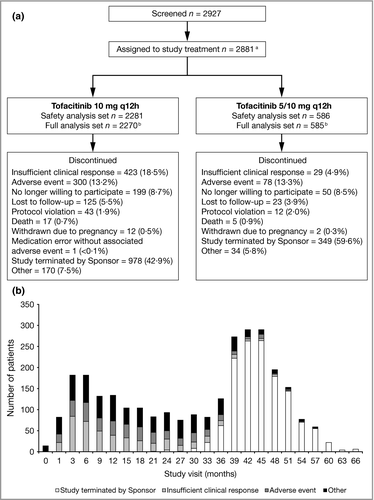
Overall, 2927 patients were screened and 2881 assigned to study treatment (Fig. 1). The safety analysis set included 2867 patients; 2855 patients were included in the FAS. The majority of patients (n = 2281; 79·6%) received tofacitinib 10 mg q12h for ≥ 80% of their days in the study and 586 (20·4%) received a variable dose.
Patient demographics and baseline disease characteristics were generally similar between tofacitinib dose groups. The median age of the patients was 46·0 years, 70·6% were male and median PASI score at baseline was 19·2 across dose groups (Table 1). However, median weight and body mass index were numerically higher in the tofacitinib 10 mg q12h group (87·0 kg and 29·0 kg m−2, respectively) vs. the 5/10 mg q12h variable dosing group (81·4 kg and 27·9 kg m−2, respectively).
| Tofacitinib 10 mg q12h (n = 2281) | Tofacitinib 5/10 mg q12h (n = 586) | Total tofacitinib (n = 2867) | |
|---|---|---|---|
| Demographic characteristics | |||
| Median (IQR) age (years) | 46·0 (36·0–55·0) | 48·0 (37·0–56·0) | 46·0 (36·0–55·0) |
| Male sex | 1641 (71·9) | 383 (65·4) | 2024 (70·6) |
| Race | |||
| White | 1948 (85·4) | 533 (91·0) | 2481 (86·5) |
| Black | 32 (1·4) | 5 (0·9) | 37 (1·3) |
| Asian | 184 (8·1) | 20 (3·4) | 204 (7·1) |
| Other | 117 (5·1) | 28 (4·8) | 145 (5·1) |
| Median (IQR) weight (kg) | 87·0 (74·8–102·0) | 81·4 (71·0–95·0) | 85·7 (73·8–100·0) |
| Median (IQR) BMI (kg m−2) | 29·0 (25·3–33·6) | 27·9 (24·7–31·6) | 28·7 (25·1–33·2) |
| Current smoker | 822 (36·0) | 213 (36·3) | 1035 (36·1) |
| Current alcohol consumption | 1237 (54·2) | 292 (49·8) | 1529 (53·3) |
| Disease characteristics | |||
| Median (IQR) PASI | 19·5 (15·4–26·3) | 18·0 (13·9–22·9) | 19·2 (15·1–25·6) |
| Median (IQR) total % psoriatic BSA | 24·0 (16·0–39·0) | 19·5 (13·0–31·0) | 23·5 (15·0–37·0) |
| PGAa | |||
| Clear | 4 (0·2) | 9 (1·5) | 13 (0·5) |
| Almost clear | 48 (2·1) | 25 (4·3) | 73 (2.6) |
| Mild | 69 (3·0) | 40 (6·8) | 109 (3·8) |
| Moderate | 1843 (80·8) | 426 (72·7) | 2269 (79·1) |
| Severe | 313 (13·7) | 86 (14·7) | 399 (13·9) |
| Missing | 4 (0·2) | 0 (0.0) | 4 (0·1) |
| Median (IQR) ISI | 6 (3–8) | 5 (3–8) | 6 (3–8) |
| Median (IQR) DLQI | 12 (7–18) | 10 (5–16) | 12 (7–17) |
| Psoriatic arthritisb | 559 (24·5) | 95 (16·2) | 654 (22·8) |
| Nail psoriasisb | 1230 (53·9) | 259 (44·2) | 1489 (51·9) |
- Baseline data were derived from the baseline visit of the qualifying study for patients who enrolled in the long-term extension (LTE) ≤ 14 days after qualifying for study participation; for patients who enrolled in the LTE > 14 days after qualifying for study participation, baseline data were taken from the start of the LTE study. Data are n (%) unless otherwise indicated. IQR, interquartile range; BMI, body mass index; PASI, Psoriasis Area and Severity Index; BSA, body surface area; PGA, Physician's Global Assessment; ISI, Itch Severity Item; DLQI, Dermatology Life Quality Index. aPatients who enrolled > 14 days after qualifying study duration may have had baseline PGA of ‘clear’, ‘almost clear’ or ‘mild’ at the LTE study baseline. bBased on medical history.
Enrolment into the LTE occurred within 14 days of completing qualifying study participation for 88% of patients. For patients with baseline values from the qualifying study, the time from baseline to the start of the LTE ranged from 12 to 56 weeks, owing to the variable duration of the qualifying studies. Previous tofacitinib exposure (either 5 or 10 mg q12h) during qualifying studies ranged from 0 to 56 weeks; some patients received only placebo in the qualifying studies. Some patients in the Oral treatment Psoriasis Trial (OPT) Compare study received etanercept in this qualifying study prior to receiving tofacitinib in the LTE.4
The median duration of treatment in the LTE was 35·6 months (tofacitinib 10 mg q12h: 32·5 months; 5/10 mg q12h: 38·9 months). By month 54, fewer than 100 patients remained in the study, as patients entered the LTE at different times, and therefore may not have reached month 54, at the time of study termination. In total, 1327 patients were discontinued as a result of study termination and 1523 patients were discontinued for other reasons (Fig. 1). Beyond month 36, the majority of discontinuations were due to the termination of the study (Fig. 1). The total tofacitinib exposure in the LTE was 7025 patient-years (tofacitinib 10 mg q12h: 5360 patient-years; 5/10 mg q12h: 1665 patient-years). Drug compliance (assessed by the number of tablets dispensed/returned at each study visit and patient dosing diaries) was high through month 66, with the mean percentage of compliance with study drug ranging from 93·3% to 98·7% with tofacitinib 10 mg q12h and from 95·4% to 99·1% with tofacitinib 5/10 mg q12h.
Safety
AEs were reported in 2366 (82·5%) patients, serious AEs (SAEs) in 392 (13·7%) and discontinuations due to AEs in 398 (13·9%; Table 2). Dose reductions from tofacitinib 10 mg q12h to 5 mg q12h as a result of AEs were reported in 49 (1·7%) patients, of whom 39 (1·4%) had events that were potentially related to tofacitinib treatment. Frequency of AEs, SAEs and discontinuations due to AEs were similar between the tofacitinib 10 mg q12h (82·2%, 13·3% and 13·9%, respectively) and 5/10 mg q12h (83·6%, 15·0% and 14·0%, respectively) groups (Table 2). The most common Medical Dictionary for Regulatory Activities (MedDRA)-defined system organ classes of AEs in the tofacitinib 10 and 5/10 mg q12h groups, respectively, were infections and infestations (57·1% and 58·5%) followed by investigations (30·1% and 32·6%), musculoskeletal and connective-tissue disorders (23·9% and 24·2%) and gastrointestinal disorders (18·1% and 24·4%). Within each of these system organ classes, the most common AEs were, respectively, nasopharyngitis (20·9% and 20·8%), upper respiratory tract infection (11·6% and 8·7%) and bronchitis (6·0% and 8·2%) for infections and infestations; blood creatine phosphokinase increase (13·0% and 15·7%), γ-glutamyltransferase increase (3·6% and 6·5%) and blood cholesterol increase (3·8% and 5·6%) for investigations; arthralgia (7·2% and 7·2%), back pain (4·2% and 5·1%) and pain in extremity (2·7% and 2·9%) for musculoskeletal and connective-tissue disorders; and diarrhoea (3·2% and 5·5%), nausea (1·9% and 2·7%), abdominal pain upper (1·6% and 2·0%) and gastroesophageal reflux disease (1·5% and 2·2%) for gastrointestinal disorders. Overall, the most common AEs in the tofacitinib 10 and 5/10 mg q12h groups were nasopharyngitis, blood creatine phosphokinase increase, upper respiratory tract infection and hypertension (7·4% and 7·5%, respectively; Table 2). Overall 2·3% of patients had cases of hypertension that were thought to be potentially related to tofacitinib treatment. One (0·04%) patient permanently discontinued the study due to an AE of hypertension.
| Tofacitinib 10 mg q12h (n = 2281) | Tofacitinib 5/10 mg q12h (n = 586) | Total tofacitinib (n = 2867) | |
|---|---|---|---|
| Patients with AEs | 1876 (82·2) | 490 (83·6) | 2366 (82·5) |
| SAEs | 304 (13·3) | 88 (15·0) | 392 (13·7) |
| Discontinuations due to AEs | 316 (13·9) | 82 (14·0) | 398 (13·9) |
| Most common AEs by preferred term | |||
| Nasopharyngitis | 476 (20·9) | 122 (20·8) | 598 (20·9) |
| Blood CPK increased | 296 (13·0) | 92 (15·7) | 388 (13·5) |
| Upper respiratory tract infection | 264 (11·6) | 51 (8·7) | 315 (11·0) |
| Hypertension | 169 (7·4) | 44 (7·5) | 213 (7·4) |
| Arthralgia | 164 (7·2) | 42 (7·2) | 206 (7·2) |
| Urinary tract infection | 148 (6·5) | 42 (7·2) | 190 (6·6) |
| Psoriasisa | 152 (6.2) | 40 (6·8) | 192 (6·7) |
| Bronchitis | 137 (6·0) | 48 (8·2) | 185 (6·5) |
| Herpes zoster | 143 (6·3) | 33 (5·6) | 176 (6·1) |
| Hypercholesterolaemia | 134 (5·9) | 30 (5·1) | 164 (5·7) |
- Data are n (%). AE, adverse event; SAE, serious AE; CPK, creatine phosphokinase. aRefers to plaque psoriasis.
Twenty-nine patients died during the study (Table S1; see Supporting Information), of whom 12 died while receiving tofacitinib or within 28 days after the last dose of tofacitinib, and 17 died more than 28 days after discontinuation of tofacitinib. Of the 29 deaths, nine were considered to be potentially related to the study drug by the assessing investigator: cardiac arrest; hepatic cancer metastatic, lung adenocarcinoma metastatic, lung neoplasm malignant and metastases to bone; hypertension; lung cancer metastatic and metastases to lymph nodes; pancreatic carcinoma (n = 2); pneumonia (n = 2); and subdural haematoma. In total, six deaths occurred as a result of malignancy (four events were potentially related to study drug, as listed above) and five deaths as a result of infection events [pneumonia, n = 4 (two events were potentially related to study drug, as listed above); infection secondary to surgery, n = 1]. Other reasons for death that were considered unrelated to the study drug by the investigators were alcoholic hepatic failure; cardiac arrest; completed suicide; coronary artery arteriosclerosis; myocardial infarction; respiratory failure; road traffic accident; sudden death; and thoracic vertebral fracture.
The incidence of AEs of special interest was generally similar between patients receiving tofacitinib 10 and 5/10 mg q12h (Table 3). Overall, serious infections (any infection requiring parenteral antimicrobial therapy or hospitalization) occurred in 85 (3·0%) patients who were receiving tofacitinib, or within 28 days after the last dose of tofacitinib, with an IR of 1·16 (95% CI 0·93–1·44). Herpes zoster infection occurred in 176 (6·1%) patients, with an IR of 2·51 (95% CI 2·15–2·91). Most cases were mild or moderate in severity; 10 cases graded as severe by the investigator were reported. Seven herpes zoster cases were classified as SAEs (defined as events requiring parenteral antimicrobial treatment, that are life threatening, or that result in hospitalization, death, persistent or significant disability, congenital abnormality, or were an important medical event), of which two were multidermatomal and all seven required hospitalization. Although IRs for serious infections and herpes zoster were numerically greater in the tofacitinib 10 mg q12h group vs. the 5/10 mg q12h group, the 95% CIs were overlapping.
| Tofacitinib 10 mg q12h (n = 2281) | Tofacitinib 5/10 mg q12h (n = 586) | Total tofacitinib (n = 2867) | |
|---|---|---|---|
| PY 5597·8 | PY 1727·6 | PY 7325·4 | |
| Serious infections | 73 (3·2) | 12 (2·0) | 85 (3·0) |
| IR (95% CI) | 1·31 (1·03–1·65) | 0·70 (0·36–1·21) | 1·16 (0·93–1·44) |
| Herpes zostera | 143 (6·3) | 33 (5·6) | 176 (6·1) |
| IR (95% CI) | 2·67 (2·25–3·15) | 1·97 (1·36–2·77) | 2·51 (2·15–2·91) |
| Opportunistic infection | 34 (1·5) | 8 (1·4) | 42 (1·5) |
| IR (95% CI) | 0·61 (0·42–0·86) | 0·47 (0·20–0·92) | 0·58 (0·42–0·78) |
| MACE | 14 (0·6) | 5 (0·9) | 19 (0·7) |
| IR (95% CI) | 0·25 (0·14–0·42) | 0·29 (0·09–0·68) | 0·26 (0·16–0·41) |
| Malignancy (excluding NMSC) | 37 (1·6) | 12 (2·0) | 49 (1·7) |
| IR (95% CI) | 0·66 (0·47–0·91) | 0·69 (0·36–1·21) | 0·67 (0·50–0·88) |
| NMSC | 41 (1·8) | 11 (1·9) | 52 (1·8) |
| IR (95% CI) | 0·74 (0·53–1·01) | 0·64 (0·32–1·15) | 0·72 (0·54–0·94) |
| Pustular, erythrodermic or guttate psoriasis | 14 (0·6) | 2 (0·3) | 16 (0·6) |
| IR (95% CI) | 0·25 (0·14–0·42) | 0·12 (0·01–0·42) | 0·22 (0·13–0·36) |
- Data are n (%) unless otherwise indicated. Incidence rates [IRs; patients with event per 100 patient-years (PY)] are based on events reported up to 28 days beyond the last dose of study drug. MACE, major adverse cardiovascular events; CI, confidence interval; NMSC, nonmelanoma skin cancer. aIncludes serious and nonserious herpes zoster events.
Events adjudicated as opportunistic infections occurred in 43 (1·5%) patients overall; 42 events occurred within 28 days of study treatment, with an IR of 0·58 (95% CI 0·42–0·78). Opportunistic infections included herpes zoster multidermatomal (n = 22), herpes zoster in two adjacent dermatomes (n = 14), disseminated herpes zoster (n = 3), invasive herpes simplex (n = 1), listeriosis (n = 1), tuberculosis (n = 1) and bartonellosis (n = 1).
Through 28 days after the last tofacitinib dose, major adverse cardiovascular events (MACE) occurred in 19 (0·7%) patients with an IR of 0·26 (95% CI 0·16–0·41). An additional six cases occurred more than 28 days after the last tofacitinib dose. Overall, MACE included nine myocardial infarction events [0·3% (one event was fatal)], 10 cerebrovascular accidents (0·3%) and seven other cardiovascular deaths (0·2%). Malignancies [excluding nonmelanoma skin cancer (NMSC)] occurred in 49 (1·7%) patients receiving tofacitinib or within 28 days of the last tofacitinib dose (IR 0·67; 95% CI 0·50–0·88), with an additional 10 malignancies reported beyond 28 days. The most common malignancies were prostate cancer (n = 15; 0·7%), lung cancer (n = 9; 0·3%) and breast cancer (n = 6; 0·7%). NMSC occurred in 52 (1·8%) patients (IR 0·72; 95% CI 0·54–0·94) within 28 days of the last tofacitinib dose, with an additional three cases beyond 28 days; some patients had more than one NMSC event, meaning that, overall, 33 (1·2%) basal cell carcinomas and 31 (1·1%) squamous cell carcinomas were reported. Six (0·3%) patients receiving tofacitinib 10 mg q12h and one (0·2%) receiving tofacitinib 5/10 mg q12h had AEs adjudicated as gastrointestinal perforation events. AEs adjudicated as interstitial lung disease were reported in five (0·2%) patients receiving tofacitinib 10 mg q12h and two (0·3%) receiving 5/10 mg q12h. There was one completed suicide (as described above), two events of suicide attempt and one event of suicidal ideation, all in the tofacitinib 10 mg q12h group.
Changes in laboratory parameters over time are presented in Figure S1 (see Supporting Information). After an initial increase from baseline, mean low-density and high-density lipoprotein cholesterol levels were generally stable through month 54. Confirmed creatine kinase elevations more than 10 times the upper limit of normal were reported in nine (0·3%) patients (tofacitinib 10 mg q12h, n = 6; 5/10 mg q12h, n = 3). No cases of renal impairment or rhabdomyolysis were reported in these patients; two were withdrawn from the study due to creatine kinase elevation. An initial increase from baseline in mean lymphocyte count was observed, followed by a gradual decrease over time. Mean neutrophil count and haemoglobin levels were stable over time in this LTE, following an initial decrease from baseline observed in the prior qualifying studies. There were no confirmed cases of Hy's Law, defined as patients with alanine or aspartate aminotransferase three or more times the upper limit of normal and total bilirubin two or more times the upper limit of normal. Vital signs and ECG reports were stable over time. A mean increase from baseline in body weight was observed over time (1·3 kg at month 3; 2·0 kg at month 12; 4·1 kg at month 54).
Efficacy
In this LTE study, efficacy observed in the qualifying studies was generally maintained to month 54, among patients who remained in the study (Fig. 2). However, patient numbers decreased substantially at later time points (n = 105 at month 54), both as a result of discontinuations due to the termination of the study, and as a result of other reasons, including 452 (15·8%) patients who discontinued owing to inadequate clinical response.
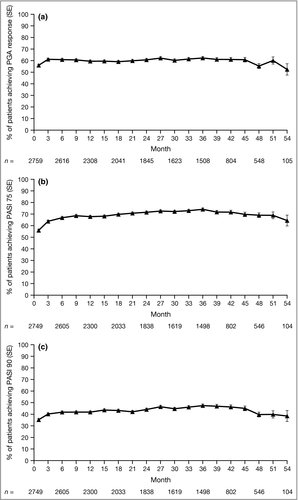
Through month 54, of those patients remaining in the study at each time point, PGA response was achieved by 52–62% of patients, PASI 75 response by 56–74% and PASI 90 response by 35–47%. PASI 50 response rates and change from baseline in PASI score were also generally sustained through month 54 (Fig. S2; see Supporting Information). Overall, 192 (6·7%) patients experienced worsening of their plaque psoriasis as determined by the investigator, and 125 (4·4%) patients had a PASI score ≥ 125% of their baseline value. As a result of worsening of psoriasis, 45 patients permanently discontinued tofacitinib treatment and five temporarily stopped treatment; some of these patients had previously stopped tofacitinib treatment at the time of their worsening psoriasis.
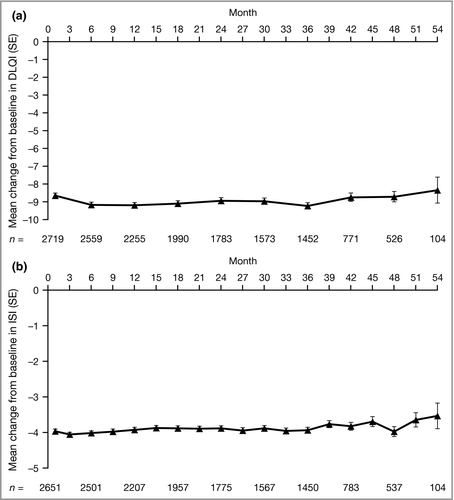
Patient-reported improvements from baseline in quality of life, assessed by the DLQI, and pruritus, assessed using the ISI, were sustained through month 54 among patients who remained in the study (Fig. 3). Improvements from baseline in other PROs (PtGA, SF-36 PCS and MCS, and EQ-5D) were also maintained from month 1 through to month 54 (Figs S3–S5; see Supporting Information).
Discussion
Owing to the chronic relapsing inflammatory nature of plaque psoriasis, long-term maintenance treatment with therapies that are effective over time without an increased risk of safety events with longer exposure are necessary.21, 22 This LTE study in patients with moderate-to-severe plaque psoriasis, who had participated in qualifying phase II or III tofacitinib clinical studies, demonstrated a safety profile that was stable for up to 66 months of tofacitinib treatment. Efficacy achieved during the randomized clinical trials was sustained through month 54 in patients who remained in the LTE. These data represent the longest report to date of efficacy and safety data for a JAK inhibitor for the treatment of moderate-to-severe plaque psoriasis.
The overall long-term safety profile of tofacitinib in this study was consistent with that observed in the phase III studies.4-7 AEs were reported in the majority of patients (82·5%) through month 66, whereas SAEs and discontinuations due to AEs were reported in approximately 14% of patients. Infections remained the most common AEs. Continuous use of tofacitinib 10 mg q12h did not appear to be associated with higher rates of AEs, SAEs or discontinuations due to AEs than using a variable 5/10 mg q12h dose. Similarly, the incidence of AEs of special interest, such as serious infections (1·16; 95% CI 0·93–1·44), malignancies (0·67; 95% CI 0·50–0·88) and MACE (0·26; 95% CI 0·16–0·41), over 66 months were generally consistent with previous reports over 33 months, including exposure in patients receiving tofacitinib 5 or 10 mg q12h in both this LTE and pivotal phase III psoriasis studies [serious infections 1·93 (95% CI 1·44–2·53); malignancies 1·15 (95% CI 0·78–1·63); MACE 0·41 (95% CI 0·20–0·73)],7 and did not appear to be more frequent in the 10 mg q12h vs the 5/10 mg q12h group.
An increased risk of herpes zoster has previously been identified among patients with psoriasis receiving tofacitinib, with risk factors including Asian ethnicity, higher tofacitinib dose, prior biological use and older age.23 The overall IR of herpes zoster over 66 months (2·51; 95% CI 2·15–2·91) reported here was similar to that reported in a previous interim analysis over 33 months including data from both this LTE and pivotal phase III studies (2·53; 95% CI 1·96–3·21),7 and suggests no increased risk of herpes zoster infection with longer exposure to study drug. Most cases of herpes zoster in this study were mild or moderate.
JAK pathways are under investigation as a potential target for psoriasis therapies due to the central role played by JAK signalling in many of the inflammatory pathways involved in psoriasis pathogenesis.24-26 In this LTE study, more than 50% of patients who remained in the study achieved a PGA or PASI 75 response at each time point. Sustained improvements in PROs were also reported, including those measuring health-related quality of life, pruritus, physical and mental health status, and overall patient assessment of their disease severity. Reductions in pruritus severity were reported in phase III studies, and exceeded the minimal clinically important difference for the ISI.27 The sustained improvement in pruritus is of particular interest, as this is one of the most bothersome symptoms for patients.28 It should be noted that there was a reduction in patient numbers over the course of the study conduct, which could limit the interpretation of efficacy results, as patients who remained in the study were likely to be those who showed efficacy and were tolerant to tofacitinib. Furthermore, many patients did not have the opportunity to participate in the study up to month 54; more than half of the patients who discontinued after month 36 discontinued owing to termination of the study. Because of the progressive decrease in patient numbers after month 36, efficacy analyses at later time points should be interpreted with caution.
This was an open-label study with no placebo or active control comparator arms, which limits conclusions. In addition, no data have been included from patients during the qualifying studies, although patients received up to 1 year of tofacitinib treatment prior to entering the LTE study. In this study, dose adjustments between 5 and 10 mg q12h were allowed at the investigator's discretion; therefore, there were no fixed-dose treatment groups for formal comparison, and no tofacitinib 5 mg q12h-only treatment group. In addition, the reasons for dose adjustments were not always provided and may have been due to either efficacy or safety reasons.
In conclusion, in patients with moderate-to-severe plaque psoriasis, the safety profile of tofacitinib up to 66 months was stable over time and consistent with that observed in prior tofacitinib studies, and no new safety signals were observed. Improvements in efficacy end points and PROs observed in the qualifying studies were sustained up to 54 months among patients remaining in this LTE study.
Acknowledgments
The authors thank the patients, investigators and study teams involved in the A3921061 study.
Appendix 1
F.V. has been a principal investigator, member of a scientific advisory board or speaker for AbbVie, Amgen, Eli Lilly, Janssen, Merck, Novartis and Pfizer Inc. N.J.K. has been an investigator, grant reviewer, speaker or advisory board member for AbbVie, Amgen, Dermira, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer Inc, Prothena, Regeneron, Sun and Valeant. R.B. has participated in advisory boards for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Merck, Novartis and Valeant. He has received consulting and/or speaking support from AbbVie, Amgen, Celgene, Eli Lilly, Galderma, Incyte, Janssen, Leo Pharma, Merck, Novartis and Pfizer Inc. He is an investigator for and his institution receives grant support from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, GSK-Stiefel, Merck, Novartis, Pfizer Inc, Kineta, Incyte, Janssen and Leo Pharma. N.B. has been a principal investigator for Amgen, Celgene, Eli Lilly, Galderma, Leo Pharma, Merck Serono, Novartis, Pfizer Inc and Regeneron. T.-F.T. has conducted clinical trials or received honoraria for serving as a consultant for AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, GSK-Stiefel, Janssen-Cilag, Leo Pharma, Merck, Novartis Pharmaceuticals, Pfizer Inc and Serono International SA (now Merck Serono International). A.T. was an employee and shareholder in Pfizer Inc at the time of the study. M.K.H., W.C.P., H.T., H.V. and A.C.G. are employees and shareholders of Pfizer Inc.




