Prospective study in bullous pemphigoid: association of high serum anti-BP180 IgG levels with increased mortality and reduced Karnofsky score†
Plain language summary available online
Summary
Background
Bullous pemphigoid (BP) is a subepidermal blistering disease characterized by autoantibodies against the two hemidesmosomal proteins, BP180 (type XVII collagen) and BP230. The multicentre prospective BLISTER (Bullous Pemphigoid Steroids and Tetracyclines) trial randomized 253 patients with BP to compare the benefits and harms between initial treatment with doxycycline or prednisolone.
Objectives
To analyse distinct autoantibody profiles for the prediction of the disease course in a well-characterized cohort of BP sera.
Methods
One hundred and forty-three patients of the BLISTER trial consented to participate in this serological study. Sera taken at baseline were analysed by (i) indirect immunofluorescence, (ii) anti-BP180 NC16A (16th noncollagenous domain) and anti-BP230 enzyme-linked immunosorbent assay and (iii) immunoblotting with various substrates. Results were then linked with clinical parameters including age, Karnofsky score, number of blisters, related adverse events and mortality.
Results
Disease activity correlated with immunoglobulin (Ig)G anti-BP180 levels but not with levels of anti-BP230 IgG and anti-BP180 IgE. High levels of both anti-BP180 IgG and anti-BP230 IgG were associated with a low Karnofsky score. The presence of anti-BP230 IgG was more frequent in older patients. Those with higher total IgE serum levels suffered from fewer adverse events. Higher IgG anti-BP180 levels were associated with an increased 1-year mortality rate.
Conclusions
Analysis of the autoantibody profile is not only of diagnostic relevance but may also be helpful in predicting the course of the disease.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in western countries, with an incidence of 13–43 per million per year1-3 and a prevalence of 259 per million in Germany.4 It mostly affects elderly patients and presents with tense blisters, erosions, erythema and severe pruritus.5 BP is immunopathologically characterized by autoantibodies against two proteins of the dermoepidermal junction, BP180 (type XVII collagen) and BP230.5 The diagnosis is made by the detection of tissue-bound and circulating autoantibodies. Linear immunoglobulin (Ig)G and/or C3 deposits at the dermoepidermal junction are seen by direct immunofluorescence (IF) microscopy of perilesional skin. Serum autoantibodies are detected by indirect IF microscopy on monkey oesophagus or human salt-split skin and by BP180-specific enzyme-linked immunosorbent assay (ELISA).6, 7 IgG autoantibodies against the immunodominant 16th noncollagenous domain (NC16A) of BP180 have been shown to correlate with disease activity and may thus be useful to monitor disease activity during the course of the disease.8, 9 In addition to IgG autoantibodies, many patients develop IgE and IgA autoantibodies against BP180.10-13
The multicentre prospective BLISTER (Bullous Pemphigoid Steroids and Tetracyclines) trial randomized 253 patients with BP to analyse the safety and effectiveness of initial treatment with doxycycline (200 mg per day) compared with prednisolone (0·5 mg kg−1 per day).14 The study found that a strategy of starting patients with BP on doxycycline was noninferior in terms of blister control at 6 weeks and safer in terms of serious related adverse events at 52 weeks when compared with a strategy of starting patients with BP on prednisolone alone.15 Here, fine autoantibody specificities and isotypes were analysed in sera taken at the initial study visit and correlated with the clinical data of this well-characterized patient cohort. The aim of the present study was to identify serological markers that might predict the clinical outcome of patients with BP.
Materials and methods
Blood samples
Serum was obtained at the initial study visit, before treatment was initiated, from patients participating in the BLISTER trial who had given written consent for this serological marker study (n = 143). Sera were stored at −80 °C until used. Inclusion criteria for the BLISTER trial were suspected diagnosis of BP in adults (≥ 18 years) with ≥ three significant blisters appearing on ≥ two body sites within the last week and positive direct or indirect IF microscopy (IgG and/or C3 at the dermoepidermal junction).14 Included in the present ancillary study were 69 women and 74 men with a mean age ± SD of 77 (± 11 years; range 40–98). Of these patients, 42 showed mild, 59 moderate and 42 severe disease at study entry. The study was performed according to the protocol of the Declaration of Helsinki and approved by the Ethics Committee of the University of Lübeck (09–134) as well as the local ethics committees of all study centres.
Clinical data
Clinical data were derived from the BLISTER study. Besides epidemiological data like age and sex, disease severity and Karnofsky score at baseline as well as disease severity and treatment-related adverse events recorded at every study visit (weeks 3, 6, 13, 26, 39 and 52), were included (see Supplementary Tables S1–S4; see Supporting Information). Disease severity was scored according to the number of blisters: mild, < 10 blisters; moderate, 10–30 blisters; severe, > 30 blisters. Karnofsky score ranges from 0 to 100 (detailed in File S1; see Supporting Information). Prospective hypotheses included the association between specificities, isotypes and levels of autoantibodies with disease severity, treatment regimen and Karnofsky score. Post hoc analyses included the association between serum biomarkers with mortality and adverse events.
Serum analyses
All 143 sera were analysed by (i) indirect IF microscopy on 1 mol L−1 NaCl-split human skin (IgG and IgA reactivity), (ii) BP180 NC16A ELISA and BP230 ELISA (both IgG reactivity; Euroimmun, Lübeck, Germany) and (iii) BP180 NC16A ELISA (IgE reactivity).16 Sera unreactive by indirect IF microscopy on salt-split skin were tested on monkey oesophagus (IgG, IgA). Sera with IgG binding to the epidermal side of salt-split skin but without IgG ELISA reactivity against BP180 NC16A were tested by immunoblotting with conditioned concentrated medium of cultured HaCaT cells (to detect IgG against LAD-1).17, 18 Sera that exhibited IgA binding to the epidermal side of salt-split skin (IgA reactivity) were also immunoblotted against this substrate and against recombinant BP180 NC16A.19 All sera with IgG binding along the blister floor by indirect IF microscopy on salt-split skin were subjected to immunoblotting with (i) extract of the extracellular matrix of cultured HaCaT cells (to detect IgG4 reactivity against laminin 332),20 (ii) extract of human dermis (to detect IgG4 reactivity against p200 and type VII collagen)21 and (iii) recombinant C-terminus of laminin γ1 (IgG4).21
Statistics
For all analysed variables, median, quartiles, ranges and percentages are given as appropriate. Due to the high number of individuals with negative or extensive values of autoantibody serum levels, values were categorized into low vs. high or in the case of BP230 negative vs. positive. Cut-offs for BP180 IgG, BP180 IgE and total IgE were 250 U mL−1, optical density 0·05 and 500 U mL−1, respectively. Whenever effects were investigated on values measured post-treatment, the treatment arm was included as a covariate in the analyses. For all comparisons, two-sided P-values are reported that are viewed as descriptive measures of evidence, and no adjustment for multiple testing was performed. Specifically, possible associations between pretreatment autoantibody serum levels and disease severity in terms of initial number of blisters were investigated using Cochran–Armitage trend tests with categorized autoantibody levels as binomial variable and number of blisters as ordinal response. Mann–Whitney U-tests were performed to analyse differences in Karnofsky scores as well as age between individuals with high vs. low autoantibody levels. To investigate the relationship between initial autoantibody serum levels on one hand and response over time in terms of ≤ three blisters and mortality on the other, logistic regression models were developed predicting favourable outcome from autoantibody levels using treatment arm as covariate. Possible associations between serum levels of total IgE and the number of at least moderate adverse events as well as specific adverse events were assessed using ordinal or logistic regression models predicting the number of adverse events from total IgE using treatment arm as covariate (see Table 1).
| Clinical features | IgG NC16A | IgE NC16A | IgG BP230 | Total IgE |
|---|---|---|---|---|
| Severity of diseasea | < 0·001 | 0·070 | 0·259 | 0·105 |
| Karnofsky scoreb | < 0·001 | 0·978 | 0·002 | 0·473 |
| Ageb | 0·200 | 0·084 | 0·016 | 0·293 |
| Mortalityc | 0·021 | 0·162 | 0·612 | 0·071 |
| Adverse eventsc | 0·681 | 0·742 | 0·122 | 0·035 |
- Ig, immunoglobulin; NC16A, 16th noncollagenous domain.
- Significant values are shown in bold.
Results
Autoantibody reactivities
Epidermal binding of IgG autoantibodies was observed in 126 of 143 (88·1%) sera by indirect IF microscopy on human salt-split skin. Five (3·5%) sera showed both epidermal and dermal binding of IgG, and two (1·4%) sera showed dermal binding only. Ten (7%) sera were negative (IgG). Four of the five sera with both epidermal and dermal binding revealed reactivity against the p200 protein and laminin γ1 by immunoblotting, respectively, as well as reactivity with BP180 by ELISA and immunoblotting. In one of the five sera, the target antigens could not be identified. In the two sera with exclusively dermal binding on salt-split skin, IgG reactivity with the p200 protein and laminin γ1 was found.
When the 10 sera unreactive by indirect IF microscopy on human salt-split skin were analysed by indirect IF microscopy on monkey oesophagus, one serum showed IgG reactivity against the basal membrane zone and one serum showed IgA reactivity against endomysium. This serum revealed IgA reactivity against tissue transglutaminase and deaminated gliadin-analogous fusion peptides by ELISA (Euroimmun). Of the remaining eight unreactive sera, two revealed IgG antibodies against LAD-1, three showed IgG reactivity against laminin γ1 (one with additional reactivity against the p200 protein), one showed anti-BP230 IgG by immunoblotting and two showed anti-BP180 NC16A IgG by ELISA.
IgA binding to the blister roof was detected in 31 of 143 (21·7%) sera by indirect IF microscopy on salt-split skin. In 25 of 29 sera (86·2%; two of the 31 sera could not be further analysed due to insufficient serum) IgA antibodies against BP180 NC16A or LAD-1 were detected by immunoblotting.
In 121 of 143 (84·6%) sera, IgG ELISA reactivity with BP180 NC16A was observed with values ranging from 20·4 to 8048 U mL−1. IgE autoantibodies against BP180 NC16A were seen in 48 of 138 (34·8%) sera (five could not be analysed due to insufficient serum). In 74 of 143 (51·7%) sera, anti-BP230 IgG was detected ranging from 22·1 to 3268 U mL−1. Elevated levels of total IgE (> 100 U mL−1) were seen in 61 of 138 (44·2%) sera. Sera that revealed the diagnosis of anti-p200/laminin γ1 pemphigoid (n = 9) or dermatitis herpetiformis (n = 1) were excluded from subsequent correlation analyses.
Association of initial autoantibody serum levels with disease activity and Karnofsky score
Pretreatment anti-BP180 NC16A IgG serum levels were associated with disease severity (P < 0·001). In contrast, no relation was observed between disease activity and serum levels of IgG anti-BP230 (P = 0·259) and IgE anti-BP180 NC16A (P = 0·070). Interestingly, higher IgG serum levels of anti-BP230 (P = 0·002) and anti-BP180 NC16A (P < 0·001), respectively, were associated with a lower Karnofsky score.
Association of initial autoantibody serum levels and treatment response and relapses
No relationship was found between the initial autoantibody serum levels (BP180 NC16A IgG, BP180 NC16A IgE, BP230 IgG) and the response to treatment defined as ≤ three blisters in week 6. Similarly, there was no association between initial autoantibody serum levels and disease activity at subsequent study visits. No difference between initial autoantibody serum levels and response to treatment in the two treatment arms was observed. No association between autoantibody serum levels and relapses was found.
Association of initial autoantibody serum levels and mortality and age
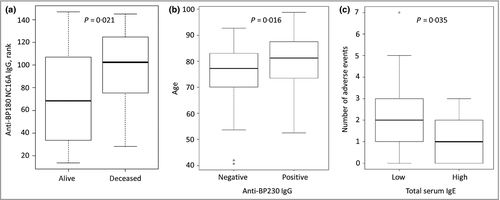
Patients with higher IgG autoantibody serum levels against BP180 NC16A showed a higher 1-year mortality rate (P = 0·021, Fig. 1a). The presence of IgG autoantibodies against BP230 was more frequent in older patients (P = 0·016, Fig. 1b). No association between age and anti-BP180 NC16A IgG nor IgE serum levels was found.
Association of initial autoantibody serum levels and adverse events
Patients with higher serum levels of total IgE seemed to develop fewer treatment-related adverse events (P = 0·035) including moderate, severe, life-threatening and fatal events during 52 weeks of observation (Fig. 1c). In a second step, the adverse events were grouped as infections, gastrointestinal or corticosteroid-associated (excluding infections) adverse events. The number of infections was identified to be negatively correlated with total serum IgE levels (P = 0·040). No correlation of total IgE serum levels with gastrointestinal (P = 0·318) or corticosteroid-associated (P = 0·054) adverse events was revealed. No association between adverse events and anti-BP180 IgG, IgE or anti-BP230 IgG was found.
Discussion
Here we analysed sera from 143 patients who took part in the BLISTER trial using several routine diagnostic assays, and related serological results with the corresponding clinical data. Following the advice of Grantham and colleagues22 in their editorial comment on the BLISTER trial, we investigated whether any baseline clinical features or serum biomarkers predict treatment response.
Because immunological inclusion criteria of the BLISTER trial comprised antibasement membrane zone reactivity by direct or indirect IF microscopy, serum autoantibodies were initially subjected to a detailed analysis for isotype and fine specificities. In four of the nine sera that fulfilled the diagnostic criteria for anti-p200/laminin γ1 pemphigoid, additional reactivity with laminin 332 and BP180, respectively, was seen, which may be explained by epitope spreading, a phenomenon already described in anti-p200/laminin γ1 pemphigoid.23 In our BP cohort, circulating antibasement membrane zone antibodies were detected in all sera. In keeping with other BP cohorts, 95% of sera stained the epidermal side of salt-split skin by indirect IF microscopy, and 91% and 56%, respectively, showed IgG ELISA reactivity with BP180 NC16A and BP230.24-29 IgE anti-BP180 NC16A reactivity was observed in 38% of BP sera, which was lower than that observed in three recent cohorts (39%, 42% and 58%).16, 30 Taken together, the serological data suggest that the present cohort is representative of a typical BP cohort.
As expected, and as previously reported in other cohorts, anti-BP180 NC16A IgG serum levels correlated with disease activity at baseline.8, 9, 16, 31 In contrast, and in line with previous studies, no association between IgE anti-BP180 NC16A levels and baseline disease severity was seen.16, 32 However, Iwata et al.13 and Hashimoto et al.30 observed a significant correlation between serum anti-BP180 IgE levels and disease severity, which may be explained by the different set-up of the ELISA systems. In contrast to the two latter studies, cut-offs of the anti-BP180 IgE ELISA used in the present study were adjusted according to total serum IgE levels, which was shown to critically affect the sensitivity of the ELISA.16 While anti-BP180 NC16A IgE levels were shown to correlate with disease activity within individuals during the course of the disease, are higher in patients with severe BP, and decrease when patients go into remission, the association between disease activity and IgE anti-BP180 reactivity appears to be lower compared with anti-BP180 IgG.12, 13, 16, 30, 33 Because the more sophisticated Bullous Pemphigoid Disease Area Index (BPDAI) was published in 2012,34 3 years after the BLISTER trial was designed, disease severity was assessed by the number of the blisters. When initiating this study, we hoped to identify a serological biomarker that predicts the response to treatment. However, the initial autoantibody specificity and isotype did not relate to the future disease course or the response to either of the two initial treatment strategies.
Karnofsky score has previously been shown to be lower in patients with BP with neurological diseases, dementia and infections.35-37 In our cohort, higher IgG anti-BP180 NC16A and anti-BP230 serum levels were associated with a lower Karnofsky score, indicating that patients with poor general condition had higher levels of circulating autoantibodies. A study by Joly et al.38 described a Karnofsky score of lower than 40 as risk factor for death in BP. While in their analysis female sex was associated with a higher mortality, this association was not evident in the present study.
The 1-year mortality rate in our cohort was 13·5%. Most studies reported 1-year mortality rates of between 19% and 41%.38-40 Lower 1-year mortality rates of 13·4% and 8·0%, respectively, were recently reported in the BLISTER study including all 253 patients with BP and a randomized controlled trial comparing dapsone and azathioprine.15, 41 In the present study, IgG autoantibody serum levels against BP180 NC16A were higher in patients that died within 1 year after diagnosis. The presence of IgG anti-BP180 reactivity has previously been related to an increased mortality.40 Thus, circulating IgG anti-BP180 antibodies may be a novel indicator for early death in BP, which needs to be confirmed in other studies. Old age, dementia and stroke have previously been identified as risk factors for death in patients with BP in a recent meta-analysis.42
In the present study, the mean age of patients was 77 years, which is representative for most BP cohorts with a mean age of between 74 and 83 years.39 Our findings of a higher detection rate of anti-BP230 IgG in older patients has not been described before. It may indicate that epitope spreading from BP180 to BP230 occurs more easily in the elderly, which may be related to the immunological senescence or altered skin structure in this patient population.
In a post hoc analysis, patients with higher total IgE serum levels were found to develop fewer adverse events including grades 2, 3, 4 and 5. This effect was shown to be mainly attributed to infections while no association with gastrointestinal and noninfectious corticosteroid-associated adverse events was seen. The mechanism underlying the observed protection from infections by high total IgE is unclear and needs to be confirmed in other cohorts.
The prospective and multicentric design and the high quality of the clinical data are strengths in this study. Limitations of the current study are that analyses were exploratory, that serum was taken only at baseline and not during the course of the disease and that only some patients of the BLISTER trial consented to this ancillary study.
Physicians in clinical practice may be aware that patients with BP with high anti-BP180 IgG may be at risk of fatal outcomes, while in those with high total IgE, fewer adverse events can be expected.
Acknowledgments
We thank Vanessa Krull for excellent technical assistance and the patients for serum donation.
Appendix:
Germany: C. Günther, Dresden; T. Luger, Münster; K. Steinbrink, Mainz; G. Wozel, Dresden. U.K.: V. Akhras, London; A. Alkali, Liverpool; A. Anstey, Newport; F. Antony, Frimley; A. Azam, Cannock; O. Aziz, Ipswich; S. Blackford, Swansea; C. Bower, Exeter; D. Buckley, Swindon; R. Charles-Holmes, Warwick; K. Davies, Barnstaple; G. Dunnill, Bristol; S. Gibbs, Swindon; R. Graham, Great Yarmouth; P. Hampton, Newcastle; K. Hussain, Lincoln; A. Ilchyshyn, Coventry; G. Kaushal, Reading; A. Layton, Harrogate; V. Lewis, Taunton; H. Malhomme, Reading; I. Nasr, Reading; A. Ormerod, Aberdeen; D. Rallan, Ipswich; J. Ravenscroft, Nottingham; I. Salvary, Great Yarmouth; D. Seukeran, Reading; D. Shipley, Carmarthen; J. Sterling, Cambridge; S. Velangi, Birmingham; V. Venning, Oxford; E. Veysey, Swansea; R. Wachsmuth, Yeovil; S. Wahie, Durham; A. Wright, Bradford.




