The setae of parasitic Liphyra brassolis butterfly larvae form a flexible armour for resisting attack by their ant hosts (Lycaenidae: Lepidoptera)
Abstract
Caterpillars of the lycaenid butterfly, Liphyra brassolis, live inside the nests of arboreal weaver ants, Oecophylla smaragdina, and eat their brood. Observations of mature larvae suggest that they are impervious to relentless ant molestation, yet they lack sclerotized cuticular plates. We document a novel form of integumental defence that imparts protection from ant attack whilst maintaining the flexibility necessary to walk with a hydraulic skeleton. Analysis of the trunk integument and cuticular structures of early and late instars of L. brassolis using light microscopy, scanning electron microscopy, and histology revealed three new setae types (disc, clavate, and lanceolate), as well as three new cuticular structures (pored sockets, cuticular pores, and cuticular domes). The unique cuticle is covered with lanceolate setae, which act as endocuticular struts, and overlapping scale-like sockets, which form a hard, flexible integument. The imperfect armour of the early-instar larvae suggests that abundant, putatively secretory pores are likely to be homologous to pore cupola organs (PCOs) found in other lycaenid larvae and thus may exude semiochemicals to allay ant aggression. The importance of these pores presumably wanes as structural (setal) cuticular defenses are reinforced in later instars, when adult ants have been observed attacking caterpillars to no avail. The caterpillar's antennae are unusual and seem to be involved in manipulating ant larvae into the caterpillar's mouth. Behavioural observations indicate that the dexterity of these structures is associated with eating ants (myrmecophagy).
Introduction
Lycaenidae (Lepidoptera: Papilionoidea) is the second-largest family of butterflies with approximately 4800 species worldwide, perhaps three-quarters of which associate with ants as juveniles (Hinton, 1951; Pierce et al., 2002). These associations range from facultative associations, in which larvae (and in some species pupae) provide intermittently attendant ant guards with nutritious secretions, to complex, obligate interactions in which larvae are never found without ants, and often only with ants from a single genus or species (Pierce et al., 2002). Hölldobler & Wilson (1990) considered the exploitation of the ants’ brood chamber the most evolutionarily advanced lifestyle known among ant parasites, perhaps because it requires cracking the ants’ semiochemical code by producing or acquiring a sufficiently similar suite of recognition signals, such as the cuticular hydrocarbon compounds used to recognize nest mates (Akino et al., 1999; Schlick-Steiner et al., 2004; Bagnères & Lorenzi, 2010).
More than 99% of lepidopteran larvae consume only living plant tissue. However, the handful of ~200 species with parasitic larvae are widely distributed throughout the lepidopteran tree of life, and have evolved from phytophagous ancestors > 30 times. Most of these lepidopteran parasites have few or no parasitic close relatives, suggesting that the habit arises frequently, but does not result in evolutionary radiation (Pierce, 1995). Shifts to parasitism are thought to have evolved in response to harsh environmental conditions (Fiedler, 1988). Some parasitic species feed on ant larvae, but many more consume ant-attended, plant-sucking Hemiptera outside ant nests (Pierce, 1995). The single largest radiation of non-plant-feeding Lepidoptera is the lycaenid subfamily, Miletinae, which includes species that feed on Hemiptera, ant regurgitations, and ant larvae (Eliot, 1973, 1986; Kaliszewska et al., 2015). Liphyra brassolis Westwood is one of these ant-eating species.
Putatively mutualistic associations between lycaenid butterflies and ants have received much attention and have been extensively studied in some species, leading to a considerable understanding of costs and benefits for both partners (Pierce & Elgar, 1985; Pierce & Easteal, 1986; Pierce et al., 1987, 2002; Fiedler, 1991; Fiedler et al., 1992). Ant workers provide enemy-free space for the vulnerable caterpillars and also act as a ‘standing guard’, defending the larvae against predators and parasites (Atsatt, 1981). Ant attendance is primarily sustained by gustatory and/or semiochemical mediation involving at least three types of ant-associated organs: the pore cupola organs (PCOs) spread across the caterpillar's trunk; the dorsal nectary organ (DNO) on the seventh abdominal segment; and the tentacle organs (TOs) on the eighth abdominal segment (Newcomer, 1912; Malicky, 1970; Kitching & Luke, 1985; Leimar & Axen, 1993; Axén, Leimar & Hoffman, 1996; Hojo et al., 2008, 2009).
All three organs are exocrine, secreting substances that entice, alarm, appease, feed, and/or manipulate attending ants (Pierce et al., 2002; Hojo, Pierce & Tsuji, 2015). The exact nature of secretions from lycaenid exocrine glands is still poorly known. For several species, the DNO has been shown to secrete droplets containing sugars and amino acids (e.g. Maschwitz, Wüst & Schurian, 1975; Pierce & Nash, 1999; Wada et al., 2001; Daniels, Gottsberger & Fiedler, 2005), and more recent work on the Japanese species, Narathura japonica Murray, has shown that the DNO secretions can also manipulate attendant ant behaviour via the dopaminergic pathway (Hojo et al., 2015). For the few species that have been examined, the PCO secretions may contain polypeptides and/or free amino acids (Pierce, 1989; Henning, 1997; Hojo et al., 2014). The TOs secrete volatile substances that attract and alert ants when a caterpillar is alarmed; however, the chemicals produced by these organs have yet to be characterized (Clark & Dickson, 1971; Axén et al., 1996; Hojo et al., 2014). The DNOs and TOs have been lost independently several times within the Lycaenidae, and lineages without them are rarely ant associated (Pierce et al., 2002). All three types of organs are typically present in myrmecophilous species and can pacify many species of otherwise aggressive and/or predatory ants.
Lycaenid immatures have a variety of other adaptations for entraining ant attendance. Some larvae and pupae produce substrate-borne vibrations that attract ants (Downey & Allyn, 1973; DeVries, 1990; Travassos & Pierce, 2000), and at least one parasitic species that lives in ant nests mimics the vibrational signals of the ant queen (Barbero et al., 2009). Many species also have dendritic setae, although the function of these is still a matter of debate (Common & Waterhouse, 1981).
Other, less obvious, modifications linked to ant association include defensive adaptations, such as reduced thrashing behaviour, which allow the caterpillar to exploit enemy-free space (Atsatt, 1981). Another adaptation to myrmecophily in the Lycaenidae is a thickened defensive cuticle, which has been described as being up to 20 times thicker than that of other caterpillars the same size (Malicky, 1969, 1970). The cuticle of myrmecophilous lycaenids is typically greatly thickened along the dorsal and lateral parts of the caterpillar, providing a mechanical defence that is contoured in such a way that it can withstand ant aggression in particular (Malicky, 1969, 1970). The thickness of lycaenid larval cuticles are variable and associated with the degree of association between ant and caterpillar, showing how attuned the myrmecophilous organs are to their host ants (Dupont, 2012). The cuticle thickness presumably represents a last resort when chemical defenses fail to appease aggression from attendant ants. Besides the thickened cuticle, a number of modified setae have been suggested to be associated with larval myrmecophily. Among the main setae types described are the club-shaped setae (Kitching & Luke, 1985; DeVries, Harvey & Kitching, 1986; Fiedler, 1988, 1992; Tautz & Fiedler, 1992), ‘dome setae’ (Kitching & Luke, 1985), mushroom-like setae (Fiedler, 1988, 1992; Tautz & Fiedler, 1992), and dendritic setae (Tautz & Fiedler, 1992). The diversity of cuticular setae within the Lycaenidae is far greater than currently documented, with a surprising variation occurring not only in the setae themselves but also in the setal sockets (S. Dupont, pers. observ.).
Regardless of the degree of ant–lycaenid interaction, the PCO is the only ant-associated organ that is present throughout the Lycaenidae (Pierce et al., 2002). Even in ‘myrmecoxenous’ associations, in which the presence of ant workers results in an enemy-free space but no active protection is provided, PCO secretions appease ants that might otherwise attack the caterpillars.
PCOs are not restricted to lycaenids that appear to be mutualistically associated with ants, and have been observed for parasitic relationships, including those of species of Phengaris (Maculinea) (Fiedler, 1998). Hence, PCOs are thought to be one of the earliest myrmecophilous adaptations and, to some extent, the most important ones (Fiedler, Hölldobler & Seufert, 1996; Pierce et al., 2002). The larvae of parasitic L. brassolis were once thought not to possess these chemically active structures, but as we show here, they may be present depending upon the larval instar, but may be sufficiently highly modified in this group that they were previously overlooked.
The caterpillars of L. brassolis are dietary specialists, feeding within nests of the ant Oecophylla smaragdina (Fabricius). The butterfly is distributed from northern India throughout South-East Asia to tropical Australia, and extends as far east as the Solomon Islands (Samson & Smart, 1980); its formicine host is also widely distributed (Azuma et al., 2006). The arboreal nests of O. smaragdina are formed by adult ants, which bind together tree leaves using silken threads spun by ant larvae (Hölldobler & Wilson, 1990). In some regions of South-East Asia, ant larvae and pupae from these easily accessible nests are harvested for human food, and L. brassolis larvae are typically consumed as well (Eastwood, Kongnoo & Reinkaw, 2010). Congeners of L. brassolis are restricted to New Guinea (Parsons, 2000), whilst a handful of species in the sister genus Euliphyra Holland are restricted to Africa, where they all feed within nests of the ant Oecophylla longinoda (Latreille) (Libert, 1995; Dejean & Beugnon, 1996). Both Liphyra and Euliphyra spend their entire larval and pupal stages inside a host ant nest. Larvae of species of Euliphyra feed on ant prey and ant regurgitations (trophallaxis), whereas Liphyra subsists entirely on ant brood. Liphyra caterpillars are oval, flattened, and slug-like with a smooth, hard cuticle that is impervious to ant bites. The tank-like morphology of L. brassolis is almost certainly linked to its brutish, myrmecophagous lifestyle. However, published descriptions of L. brassolis focus on later instars, and little is known of the first three instars. This study presents a detailed morphological study of the extraordinary carapace-like trunk cuticle of L. brassolis in both early and late instars.
Material and Methods
We examined 20 larval L. brassolis specimens. Two early-instar and four later-instar voucher specimens from Mt Stuart in tropical Queensland, Australia, were preserved in strong ethanol and deposited at the Museum of Comparative Zoology, Harvard University. An additional 14 specimens from Townsville, Queensland, Australia, were air-dried and vouchered at the Natural History Museum, London. These dried specimens comprised three early-instar larvae and 11 later-instar larvae, four of which were actually pupae encased in a final-instar larval cuticle. Interestingly, this species forms a puparium by pupating within its final-instar larval exuvium, presumably as an additional defence against ants (Cottrell, 1987).
Conventional light microscopy, scanning electron microscopy, and histology of paraplast-embedded material were used to study these specimens. For conventional light microscopy, a Leica M125 stereomicroscope with a Canon DSLR (EOS 500D) was used. Specimens for environmental scanning electron microscopy (LEO 1455VP; Zeiss) were observed without further treatment, whereas specimens for study using conventional scanning electron microscopy (JSM-6335 F; Jeol) were critical point dried (CPD 030; Bal-Tec), mounted on stubs, and coated with 15 nm gold (high-resolution fine coater, JFC.2300HR; Jeol). The four most terminal segments of a wet, early-instar larva were embedded in paraffin after dehydration by immersion in a graded series of alcohol, with a final immersion in xylene. Sections of 7 μm were made using a Leica 2045 multicut microtome and stained using Mallory's trichrome stain. After staining, the tissues were mounted using cytoseal 60 (Richard-Allan Scientific) and imaged using a compound microscope (DM1000; Leica).
Interpretations of our histological and external morphological examinations were made in light of observations of live animals in the field and on video.
Results
Gross morphology
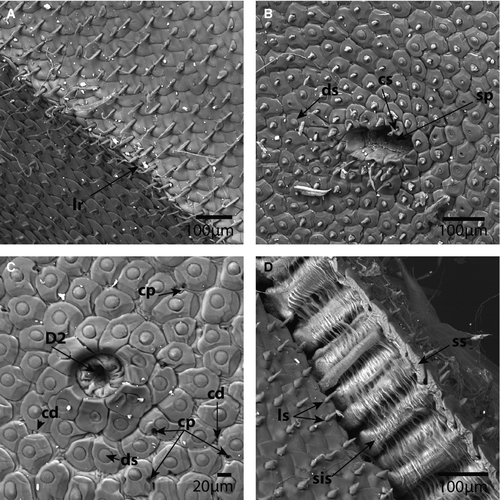
The caterpillars were ovate in dorsal view (Figs 1A, 2A). All instars were slightly widened posteriorly and had three dorsal grooves that divided the body into four parts. The anterior part included the thorax and abdominal segments I–III, followed by segment IV, segment V, and then segments VI–VIII. The posterior-most groove was slightly curved anteriorly (Figs 1A, 2A). The vestiges of a DNO were observed as a demarcated region in all instars. The cuticle within the demarcation of the DNO area was, however, similar to the surrounding cuticle, but the setae were more densely arranged (Figs 1E, 2E).
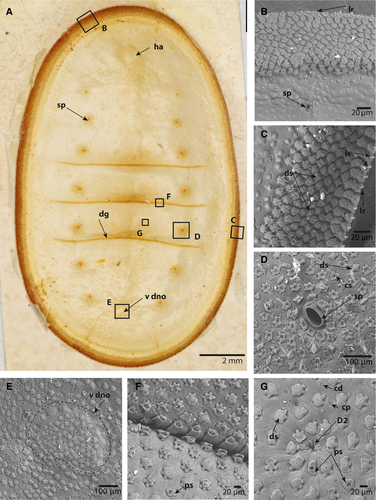
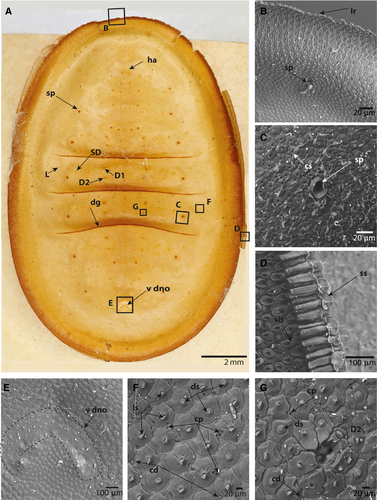
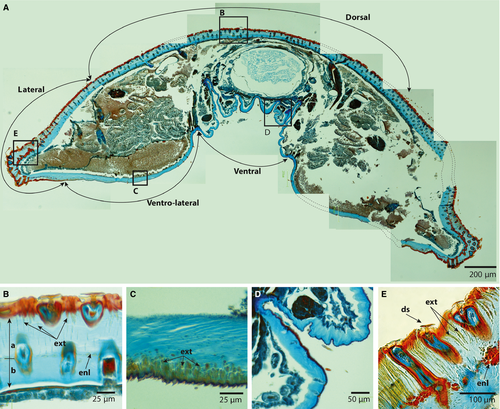
Figure 3 shows the paraplast-embedded sections of an early-instar larva treated with Mallory's trichrome stain, rendering the exocuticle red and the endocuticle blue (Vincent, 1990). The fully sclerotized cuticle does not stain and retains an amber colour. The sections containing setae (Fig. 3B, E) show that the seta and the flange of the socket were sclerotized and that the exocuticle of the sockets was embedded deeply in the underlying endocuticle. Furthermore, the exocuticle embedded in the endocuticle appeared to have unstained amber walls (Fig. 3E). The lateral and dorsal endocuticles were much thicker than the ventral endocuticle, and appeared to comprise two layers separated by an endocuticular layer (Fig. 3B, E). The ventral cuticle was approximately 40 μm, whereas the lateral and dorsal cuticles were up to 180–200 μm. The dorsal and lateral exocuticles appeared to have tendrils that extended into the endocuticle (Fig. 3B, E). These exocuticular tendrils were only visible in the more undulating exterior layer of the endocuticle, and in some places formed a red-stained intracuticular layer (Fig. 3D). The subventral cuticle also had exocuticular tendrils, but these only extended about a third into the endocuticle (Fig. 3C).
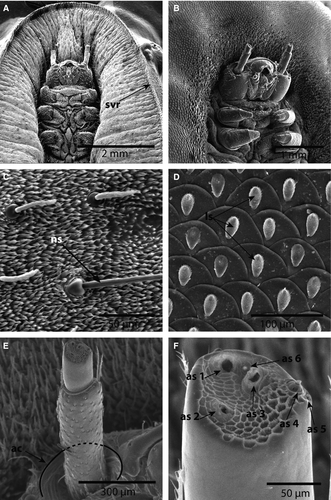
Images of the ventral region, including the head of both early and late instars, showed the peculiarities of the antennae (Figs 4E, F). The antennae appeared to be two-segmented, with the first segment being at least twice as long as the second distal segment (Fig. 4A, B, E, F). A large, cranial socket, the antacoria (ac), was situated at the base of each antenna, with cuticle that appeared flexible/eversible (Fig. 4E). Images of early instars show the antenna eversed and of different lengths, whereas Figure 4B shows the antenna positioned deep in the cranial socket. The apex of the distal segment is sculptured and has six distinct structures: three that appear to be pores or sensorial pits (as1–3); two that are structures set in undifferentiated sockets (as4–5); and a single minute dome (as6) (Fig. 4F).
Chaetotaxy
In all stages examined, four distinctly differentiated setae types and two cuticular structures were identified (Figs 1, 2, 5). Normal socketed, filiform setae (ns) were also observed on the ventrum of all larval stages (Fig. 4C).
Primary setae
Primary setae in the dorsal cuticle are reduced to invaginated, soft membranes without a socket, as illustrated by the primary seta D2 (Fig. 1G). In the early instars, the primary setae are only visible under scanning electron microscopy. In the later instars, however, the positions of primary setae D1 and D2, a sub-dorsal (SD) setum, and a lateral (L) primary setum are clearly visible as a dark ring, even to the naked eye (Fig. 2A). The dark rings around the primary setae in the later instars were observed to be the sockets of surrounding setae that form an elevated, circular crest, as illustrated by the invaginated D2 setae (Figs 2G, 5C).
Disc setae
Disc setae appear to be the most common and diverse type of seta in all instars. In early instars, the setal sockets are approximately 20–25 μm wide, irregular and dentate with four to six lateral protrusions and a central setum (Fig. 1C, D, G). In later instars, the sockets expand (50–60 μm), the shape of the sockets becoming defined by the adjacent sockets (Figs 2F, G, 5B). The setae are disc shaped and appear to retain a diameter of 20 μm in all instars. The disc setae cover the entire dorsal, lateral, and subventral body cuticle, with the exception of the crest of the lateral ridge and a region around the spiracles (Figs 1C, D, 2B–D, 5B, C). The crest of the lateral ridge in all instars is made up of an increasing number of lanceolate setae (see below), whereas the region around the spiracles in all instars is made up of clavate setae (see below).
Lanceolate setae
Lanceolate setae, which appear to be most numerous on the lateral ridge and ventral cuticle of the late instar, are distinctive in having a spearhead or lancolate shape. In the early instars, the lanceolate setae are present in a double row at the tip of the lateral ridge. The sockets resemble those of the disc setae. The setae are perpendicular to the sockets (Fig. 1C). In the late instars, and especially in the final instar, the lanceolate setae make up the entire lateral ridge and ventral cuticle, forming rows with the sockets overlapping like scales and the setae lying flat against the sockets directed towards the lateral ridge (Figs 2B, D, 4D, 5A, D). In scanning electron microscopy images with broken integument, the long tubular, sclerotized sockets embedded into the endocuticle are exposed (Figs 2D, 5D). The endocuticular socket extensions are approximately 100 μm long in late instars and almost 200 μm long in the last instar. Furthermore, the interior extension appears to terminate in a slightly bulbous enlargement.
Clavate setae
Clavate setae are characterized by having a slender body that expands terminally to form a clubbed end. The sockets are identical to those of the disc and lanceolate setae. Clavate setae are only present around the spiracles (Figs 1D, 2C, 5B).
Normal setae
Normal setae were also observed on the ventral integument. These setae have circular exterior sockets and a filiform setal shaft (Fig. 4C).
Pored sockets
Pored sockets have a cuticular socket structure similar to that of the disc, lanceolate, and clavate setae, but contain no setae. The pore is approximately 10 μm wide, slightly pear shaped and positioned centrally in the socket. Compared with the frequency of the dorsal setae with similar sockets, the pored sockets are rarer, but appear to be more frequent in the early instars (Fig. 1F, G).
Cuticular pores and cuticular domes
Dispersed among the setae sockets are cuticular pores and cuticular domes. These are simple structures that appear to be embedded in the integument without any sockets or differentiated structure around them. The cuticular pores are simple holes in the cuticle that extend through the cuticle to the basal lamina. If a pore is observed next to a socket, the socket will invariably have a deformation so that it does not obstruct the pore (Fig. 2F, G). The cuticular domes are small, convex, dome-like protrusions, approximately 8 μm wide (Figs 1G, 2F, 2G, 5C). Unlike the cuticular pores, the surrounding setae sockets do not become deformed if there is a cuticular dome next to them. The cuticular pores and domes were not observed on the subventral and ventral integument (Fig. 4C, D).
Discussion
The microscopic structures of the cuticle of L. brassolis appear to represent a previously undocumented means of providing tough, but flexible, integumentary protection. In most insects, mechanical protection is achieved by having large sclerites. Hard, chitin sclerites are well suited for protection against external forces, as they are highly stress-resistant with a high Young's modulus (Vincent & Wegst, 2004). Young's modulus is a quantity used to characterize the stiffness of an elastic material by using the ratio between stress, as defined by the force per unit area along an axis, and strain, which is the ratio of deformation over initial length. Despite these advantages, they restrict movement, requiring elastic joints between them. The soft intersegmental membrane of most insects has a Young's modulus 106 times smaller than that of sclerotized cuticle. Unlike most adult insects, which rely on their long, segmented appendages for movement, caterpillars have a hydrostatic skeleton and require flexibility for locomotion (Casey, 1991; Trimmer & Issberner, 2007). Large, interlocking sclerites would therefore hinder their mobility. The setal sockets of L. brassolis, with deep exocuticular support structures, represent a different cuticular armour that provides both flexibility and a high Young's modulus. Pressure applied by a structure larger than the gap between individual setae will be applying that pressure to multiple cuticular sockets. The sockets would then function as struts, spreading the pressure over a wider area, relieving stress on the underlying integument. In late instars and in the larval exuvia of the last instar, the largest distance subtended by a straight line between two setal sockets is no more than 25 μm, and – at the lateral ridge and subventral cuticle – the underlying integument is entirely protected by overlapping the scale-like sockets. As such, the unusual cuticle design of L. brassolis provides a strong mechanical barrier, much like that of sclerites but with more overall flexibility. This is especially true for the lateral ridge, in which the setae sockets overlap, creating scaly armour with no exposed integument.
The lateral ridge is not the only area to be fortified against host aggression. The setal sockets around the primary setae and the spiracles become progressively more raised and more closely interlocked as the larva matures. The defensive design of late-instar L. brassolis, although unique, is in overall agreement with previous descriptions of a well-armoured carapace-like structure (Fiedler, 1991). However, the observed ontogeny of the cuticle from early to last instar, and the presence of pored sockets and cuticular pores (as described below), suggests that L. brassolis is not as heavily armoured by this integumentary system while it is small and presumably more vulnerable to predation, suggesting that it may possess some form of chemical camouflage in early instars, despite previous speculation to the contrary.
The most noticeable differences between the early- and late-instar larvae are the amount of exposed integument between adjacent setal sockets, the absence of lanceolate setae on the subventral integument of the early instar, and the absence of socket crests around the dorsal primary setae and the spiracles. In the early instars, the largest gap between two sockets (measured by a straight line) is more than 180–200 μm. The integument is therefore sufficiently exposed for the mandibles of O. smaragdina (Roux et al., 2010) to reach the soft integument under the setal protection. If the amount of exposed integument is a reflection of how armoured the larval cuticle is, the early instar is less armoured and more vulnerable to ant aggression, especially when considering the subventral cuticle, and it seems reasonable to suspect that other mechanisms are in place to deter ant aggression towards younger instars.
Early instars appear to have a higher number of pored sockets and cuticular pores than the late instars. Because of their porous nature, both of these structures might be associated with secretion of chemical cues involved in ant-association. However, the basal lamina was not intact in all specimens studied because of improper fixation when collected, and it was not possible to observe any glands associated with the porous structures. Therefore, further study will be needed to confirm whether or not these porous structures have a secretory function. If they are indeed producing chemical compounds fostering adoption by ant colonies, then these structures are likely to be highly modified pore cupolae, and early instars might coexist with ants in their nests through semiochemical subterfuge, with later instars relying more heavily on mechanical defence. Because of their rarity and inaccessibility inside the nests of aggressive, acid-spraying ants, the few observations of L. brassolis larvae document aggression by their ant hosts, but these all involve later instars, which are easier to spot in the seething mass of ants that greets an entomologist tearing open an arboreal O. smaragdina nest (Chapman, 1902; Dodd, 1902; Johnson & Valentine, 1986; Cottrell, 1987; Eastwood et al., 2010).
Moreover, the eggs of L. brassolis are < 2 mm across (Braby, 2000), and the tiny first instars would presumably be an easy meal for a pugnacious ant. This is yet another reason why the youngest instars, with their small size and imperfect setal defence, are likely to be defended in different ways against ant aggression before the full deployment of the setal defence shield in later instars. Preliminary gas chromatography–mass spectrometry (GC-MS) analyses have failed to find similarity between the suite of cuticular hydrocarbons (CHC) coating mature L. brassolis larvae and those of their O. smaragdina workers or brood (D. J. Lohman and N. E. Pierce, unpubl. data); some degree of similarity in CHC profiles is a hallmark of chemical mimicry in social parasites (Bagnères & Lorenzi, 2010).
The presence of possible ant-associated structures in Liphyra and the difference in the number of structures in the early instars, coupled with an apparently more heavily armoured later instar, suggests a possible behavioural shift from an early, inquilinous form to a mature, explicitly parasitic form, much like the behavioural shift described for Phengaris (Maculinea) spp. (Elmes, Wardlaw & Thomas, 1991; Schönrogge et al., 2004). The shift in degree of ant association might also help to explain how the early instar infiltrates and survives inside its host's nest.
The presence of two kinds of porous structures that possibly mediate ant associations via semiochemicals suggests that the description of L. brassolis as missing pore cupolae (Hinton, 1951; Johnson & Valentine, 1986) could be mistaken. Unfortunately, the early-instar larva used for histology was too poorly preserved to document the internal structure of the pore setae and the cuticular pores.
The scanning electron microscopy analysis provided a glimpse of the antennal morphology of early and late instars (Fig. 4E, F). Field observations of L. brassolis and Thestor yildizae Koçak suggest that the antennae of myrmecophagous Lycaenidae can, to some extent, be involved in capturing and handling ant brood (N. E. Pierce, pers. observ.). The use of the antennae as food manipulators in these two miletine caterpillars seems to be convergent, although closer observations of additional miletine species are required. Video recordings of L. brassolis suggest that the head senses ant brood, and the antennae are used to pull the brood under the ‘hood’ concealing the head, so the ant larvae can be consumed without molestation from the adults, which seem to be trying to overturn the larvae to gain access to its soft, and presumably vulnerable, underside, which is held firmly to the substrate.
The morphology of the antenna is unusual in the absence of the off-centre third segment and the absence of the hollow, thick-walled sensilla trichodea that are usually present (Dethier, 1941). The larval antennae of Lycaenidae are described as having three-segmented antennae with the third segment highly reduced (Dethier, 1941). In L. brassolis, only two segments are observed; however, the centred position of the second segment and the large number of sensilla on the second segment suggests that the antenna consists of just the first and second antennal segments, whereas the third segment – which still bears sensilla – is reduced.
The antennal morphology and observed sensory structures are not enough to substantiate the use of the antenna as brood manipulators. However, the elongated first antennal segment, where the controlling musculature is situated (Dethier, 1941; Hasenfuss & Kristensen, 2003), as well as the large antacoria and the flexible/eversible integument, fits with the idea of a mobile appendage. The terminal second segments have six observed sensorial organs with unknown function. The only clear observation of the senor organs is that they are all of a very low profile, allowing the sculptured surface of the antenna to make contact with the brood. The use of the antenna as food manipulators is considered unique and merits future study, including observations of feeding, microanatomy of the sensory structures, and electrophysiology of the sensilla. Observations of another miletine larvae, using their antennae to manipulate food, suggests that antennal specializations could be linked to entomophagy and could be used to further the understanding of the transition to myrmecophagy in lycaenid larvae.
Acknowledgements
This paper is dedicated to the memory of our good friend and mentor, Niels Peder Kristensen, who first suggested that we work together on this fascinating topic. We thank Rod Eastwood for comments on the manuscript as well as his advice about collecting specimens of L. brassolis in the field. Mark Elgar provided support throughout the project, and Joaquin Baixeras, John Allen, and an anonymous reviewer made numerous suggestions that greatly improved the manuscript. Darlyne Murawski generously lent her photographic acumen to several scanning electron microscopy photo shoots. Funds were provided by the Villum Kann Rasmussen grant from the Carlsberg Foundation at the Natural History Museum of Copenhagen to STD, a postgraduate fellowship from the University of Melbourne and ARC DP120100162 (to Mark Elgar) to DSZ, a Putnam Expedition grant from the Museum of Comparative Zoology and a STAR Graduate Fellowship from the US EPA to DJL, and NSF DEB-9615760 to NEP. We are grateful to the Natural History Museum, London, for providing bench space to STD, and to the Australian National Insect Collection and the Yale Peabody Museum for the loan of several lycaenid larval specimens for comparative research.




