Impact of Activation Therapy for Inpatients With Major Depression: Primary and Secondary Outcomes From a Randomised Controlled Trial
Funding: This research was funded by a project grant from the Health Research Council of New Zealand (HRC NZ, grant reference: 18/152) and a project grant from the Canterbury Medical Research Foundation (CMRF). The HRC NZ and CMRF had no further role in any aspect of the study. KMD would like to acknowledge HRC NZ for fellowship funding (Sir Charles Hercus Health Research Fellowship).
Katie M. Douglas and Zoe A. Odering co-joint first authors.
ABSTRACT
Introduction
Inpatient depression is associated with high morbidity and significant cognitive impairment. Inpatient treatment often focuses on short-term stabilization with medication. Readmission rates are high. We examined the impact of a novel psychological intervention, activation therapy (AT, Behavioural Activation combined with Cognitive Activation), versus treatment as usual (TAU) on readmission rates, and cognitive, functional, and depression outcomes, in inpatient depression.
Method
A randomised controlled trial in adults hospitalised with a major depressive episode. Inpatients were randomised to AT (8 individual sessions over 2 weeks) or not (TAU). Key time points were baseline (on admission) and 14 weeks after baseline. The primary outcome was psychiatric hospital readmission rates within 12 weeks of discharge. Secondary outcomes were cognition, general functioning, depression, and ‘deactivation’ symptoms (change from baseline to 14 weeks).
Results
Ninety-seven individuals were randomised to AT (n = 47) or TAU (n = 50). Readmission rates did not differ between treatment arms (34% vs. 40%; OR = 0.76, CI = 0.30–1.90). Significant improvements for verbal learning and memory (d = 0.42) and general functioning (d = 0.58) were in favour of the AT versus TAU arms. Per protocol analysis showed additional significant effects of AT on psychomotor speed (d = 0.64) and clinician-rated depression symptoms (d = 0.56). No significant effects were observed for other secondary outcomes (subjective cognition, self-reported depression symptoms, and deactivation symptoms).
Conclusions
The AT intervention showed durable, pro-cognitive effects. Further adaptations of AT, such as the addition of maintenance sessions as patients transition to community-based care, need exploring.
1 Introduction
Inpatient depression is associated with high morbidity and risk of suicide, as well as impairment in cognitive function [1-3]. Cognitive impairment often persists after the resolution of mood symptoms [4, 5] and can cause difficulties after discharge, including in psychosocial and occupational domains [6, 7]. Readmission rates are high for those hospitalized with depression. The inpatient period, thus, provides an opportunity for intensive psychological and pharmacological treatment to address these issues.
Psychomotor slowing is a feature of severe, inpatient depression [8, 9]. Deactivation symptoms in depression—loss of interest, lack of enjoyment, indecisiveness, and reduced activity—are associated with poorer treatment responses to antidepressant medication [10] and are particularly prevalent in inpatient depression [11].
We therefore developed an intensive, brief ‘activation therapy’ (AT), combining behavioural activation (BA) and Cognitive Activation [12], delivered during the inpatient period to target directly behavioural and cognitive deactivation.
1.1 Aims
The primary aim was to investigate the impact of AT versus treatment as usual (TAU) on hospital readmission rates (within 12 weeks of discharge) for inpatients with depression. Secondary aims were to examine the impact of AT on cognition, functioning, and depression symptoms.
2 Materials and Methods
2.1 Study Design
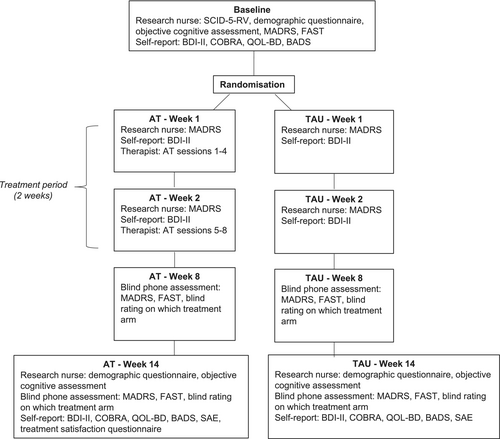
This was a prospectively registered (Australian New Zealand Clinical Trials Registry [ANZCTR], ACTRN12617000024347p), single-blinded, randomised controlled trial (RCT) comparing 2 weeks of AT with TAU for inpatients with depression, at four sites in New Zealand (see Data S2). Study recruitment began on 04/02/2019, and the final patient was recruited on 27/07/2023. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013. All procedures involving patients were approved by the New Zealand Health and Disability Ethics Committees (Northern B; reference 18/NTB/75) on 01/06/2018. The timeline for study design and assessment is presented in Figure 1. Reporting follows Consolidated Standards of Reporting Trials (CONSORT) guidelines [DATA S1, 13]. Detailed methodology of the trial is found in the protocol paper [12].
2.2 Participants
Inpatients were approached by ward staff within 2 days of admission. Patients were eligible if they had a primary Diagnostic and Statistical Manual of Mental Disorders—5th Edition (DSM-5) diagnosis of major depressive episode (MDE; unipolar or bipolar), were aged between 18 and 65 years, and were able to complete questionnaires and therapy in English. Written informed consent was obtained from all individuals. Recruitment was from four adult inpatient mental health services in New Zealand (see Data S2).
Exclusion criteria were: primary diagnosis of schizophrenia; current severe substance dependence; serious comorbid endocrinological, neurological, or chronic medical conditions; previous serious head injury (loss of consciousness ≥ 1 h); pregnancy; having completed a course of cognitive remediation (CR) in the past 24 months or electroconvulsive therapy (ECT) in the past 6 months.
2.3 Randomisation
Patients were assigned to AT or TAU following completion of the baseline assessment. The study biostatistician (CMF) completed computerized permuted block randomization prior to the study commencement. Randomization was stratified for mood disorder type (major depressive disorder [MDD] or bipolar disorder [BD]) and study site. Randomization assignment was placed in sequentially numbered envelopes, which were kept in a locked cabinet by an independent research coordinator. These envelopes were distributed sequentially to study nurses after the baseline assessment.
2.4 Interventions
2.4.1 Treatment as Usual
The control treatment was TAU on the inpatient ward. This consisted of pharmacotherapy as prescribed by the treating psychiatrist, nursing care, regular review with medical staff, and educational, diversional, and therapeutic group activities led by an occupational therapist. Psychology input was provided in a limited capacity due to resourcing constraints and would usually only occur when an inpatient's stay had become prolonged, at which point patients would no longer be receiving treatment in the study.
2.4.2 Activation Therapy
Activation Therapy was developed to address cognitive impairment and deactivation symptoms (e.g., loss of interest, diminished activity, indecisiveness [10]). It consisted of a BA component and a Cognitive Activation component (see protocol paper [12]). Both components were integrated into each therapy session. The treatment period was 2 weeks, with 8× 30–40 min, in-person AT sessions with a therapist, and a recommended 10 online cognitive practice sessions. This was additional to TAU. Therapists were registered mental health nurses who were familiar with working in an inpatient environment.
2.4.2.1 Behavioural Activation
In the BA component of AT, drawn from Lejuez et al. [14], patients were encouraged to re-engage in activities that were previously or potentially rewarding. With the therapist, patients scheduled valued, pleasant, and mastery events for completion between therapy sessions.
2.4.2.2 Cognitive Activation
Patients used a computerized cognitive program, Scientific Brain Training Pro (SBT Pro; https://www.scientificbraintrainingpro.com/) with therapist support. Tablet computers were provided on the inpatient ward to allow inpatients access during their treatment period and through which therapists could monitor their progress. Therapists selected SBT Pro exercises from a core battery of 10 exercises, depending on the patient's level of cognitive functioning. The therapist assisted the patient in practicing collaboratively during therapy sessions, and patients were encouraged to practice exercises regularly between sessions. SBT Pro exercises have an in-built algorithm that maintains the difficulty level at an 80%–90% success rate, to maximize engagement and motivation.
As part of the broader AT intervention, patients were working on a daily schedule of activities for the BA component, which included scheduled cognitive practice sessions.
2.4.2.3 Activation Therapy Fidelity and Training
Activation Therapy was delivered according to our AT manual by 5 registered mental health nurse therapists. All therapists were trained by clinical psychologists with extensive experience in training and delivery of BA (JJ) and Cognitive Activation (KMD). With regard to fidelity of AT, therapy sessions were audio-taped and 10% were randomly selected and rated to ensure adherence to therapy protocols using checklists for key components of each therapy (BA: Quality of Behavioural Activation Scale—Short Form [15]; CA: Quality of Cognitive Activation, developed by KMD). Therapists all participated in fortnightly group supervision, led by the same clinical psychologists who delivered therapy training (JJ, KD).
2.5 Study Procedure
Figure 1 presents the full study timeline. This section describes those procedures relevant to primary and secondary outcomes reported in this paper. Patients entered the study within 1 week of admission to the hospital. Baseline assessment included the Structured Clinical Interview for DSM-5 Disorders, Research Version (SCID-5-RV [16]), a demographic questionnaire, the Montgomery Åsberg Depression Rating Scale (MADRS [17]), the Functioning Assessment Short Test (FAST [18]), and a cognitive assessment (see Objective Cognitive Assessment section below). Patients also completedthe Beck Depression Inventory—Second Version (BDI-II [19]), the Cognitive Complaints in Bipolar Disorder Rating Assessment (COBRA [20]), the Quality of Life in Bipolar Disorder (QoL.BD [21]), and the Behavioural Activation for Depression Scale—Short Form (BADS-SF [22]).
Treatment, according to randomisation, commenced within 3 days of study entry and baseline assessment, and lasted for 2 weeks. At Week 14 (12 weeks after treatment-end), the same measures were conducted (see Figure 1), as well as a Serious Adverse Events Questionnaire. At 14 weeks, a blind assessor (Research Assistant) completed the MADRS and FAST via telephone (see Data S2 for assessment of blinding process).
2.6 Outcomes
2.6.1 Primary Outcome
The primary outcome was psychiatric hospital readmission rates within 12 weeks of discharge from the hospital, in the AT arm compared with TAU. This was determined from the patients' electronic hospital records.
2.6.2 Secondary Outcomes
All outcomes reported here were conducted at baseline and at the 14-week time point (see protocol paper for measure details [12]).
2.6.3 Objective Cognitive Functioning
Cognitive assessments were conducted by a Research Nurse, trained by a clinical psychologist with expertise in cognitive assessment (KMD). The choice of cognitive measures was based on those used in our previous studies of inpatient depression, which have shown sensitivity to clinical state, and recommendations of the International Society for Bipolar Disorders (ISBD) Task Force on Targeting Cognition [4, 23]. To minimise practice effects, cognitive measures were selected to avoid tasks directly analogous to the computerised cognitive exercises on SBT Pro. Key outcomes were changes in domain scores for: verbal learning and memory, visuospatial learning and memory, executive function and attention, and psychomotor speed. “Global cognition” was also calculated, which was a composite score comprising all cognitive tests. Cognitive tests were grouped into cognitive domains as follows.
- Rey Auditory Verbal Learning Test—total learning, immediate, and delayed recall (RAVLT [27])
- Brief Visuospatial Memory Test—Revised—total learning, delayed recall (BVMT-R [28])
2.6.4 Other Secondary Outcomes
Secondary outcome measures (see Figure 1) included: COBRA, FAST, QoL.BD, MADRS, BDI-II, BADS, and an “activation composite” score, derived from items from the MADRS and BDI-II (as per Uher et al. [10]).
2.6.5 Treatment Satisfaction
Patients in the AT arm completed a self-report questionnaire at 14 weeks, in which they rated how valuable they found the content of the therapy sessions and the therapeutic relationship on a scale from 1 (‘not at all valuable’) to 7 (‘very valuable’). Satisfaction with the computerised cognitive exercises was also assessed on a 1–5 scale.
2.7 Changes to Protocol
- Minor changes to cognitive tests
- Changes in recruitment sites
- Removal of ECT exclusion criteria
2.8 Statistical Analyses
Power was based on historically available 12-week inpatient readmission rates (40%) in Canterbury between 2013and 2015. To have 80% power to show a proposed clinically significant 20% reduction between groups as statistically significant (p < 0.05), 82 patients per group were required—hence a target of 165 participants [12]. All statistical tests utilized a two-tailed p-value of < 0.05 to indicate statistical significance.
- an intention-to-treat (ITT) approach, where missing data was considered as no change from baseline, and
- ‘per protocol’ analysis, with only those who completed the treatment per protocol, defined a priori as completing at least five AT sessions plus at least 90 min of cognitive exercises, as well as 14-week follow-up measures [12].
2.8.1 Primary Outcome
Logistic regression with a binary outcome of readmission or no readmission to psychiatric hospital during the 12-week post-discharge (from hospital) follow-up period was conducted, with both ITT and per protocol samples. Per protocol analysis for the primary outcome included those patients who completed the pre-defined adequate dose of therapy (as above). Completion of the 14-week assessment was not required for per protocol analysis, given the primary outcome was based on clinical records only. Mood disorder diagnosis (MDD, BD) and site were entered as covariates.
2.8.2 Secondary Outcomes
Secondary outcomes were analysed using general linear models. Change in outcomes from baseline to 14-week follow-up between AT and TAU arms were analysed using univariate ANCOVA, with treatment arm and strata (mood disorder diagnosis, study site) as fixed factors (covariates). For cognitive outcomes, z-scores for change on each cognitive variable between baseline and 14-week follow-up were calculated (difference from the mean of the full sample divided by the standard deviation of the full sample, as per Douglas et al. [29]). Cognitive domain scores were calculated by averaging the test variable z-scores relevant to that domain. The Global Cognition z-score was calculated by averaging the four cognitive domain scores. For subjective cognition, functioning, mood and deactivation measures, change scores were calculated by subtracting raw scores at 14-week follow-up from raw scores at baseline. All scores were computed such that a positive score reflected better performance.
3 Results
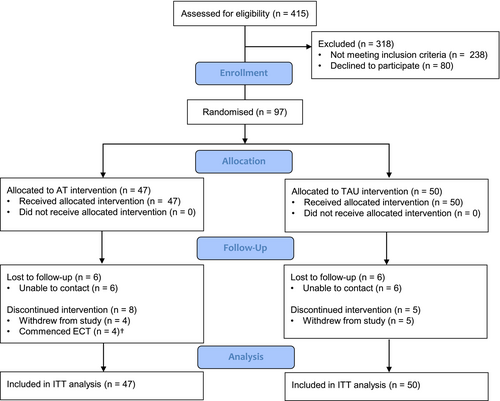
3.1 Participant Flow and Missing Data
Ninety-seven participants were recruited and randomised to AT (n = 47) or TAU (n = 50). Participant flow is presented in the CONSORT diagram (Figure 2). All inpatients were included in the ITT analyses except where there was no baseline measurement and the outcome of interest was change from baseline to follow-up. No significant difference in drop-out rate between treatment arms over the 14-week study period was found (AT, n = 14 [29.7%]; TAU, n = 11 [22.0%]; χ2 = 0.18, p = 0.67). Sixty-one patients completed the 14-week research assessments as well as the pre-defined therapy dose as described above (per protocol sample: AT, n = 25; TAU, n = 36).
3.2 Group Characteristics
Baseline demographic and clinical characteristics are presented in Table 1, and baseline scores on secondary outcomes in Data S3. The majority of the sample had a primary diagnosis of MDD (82%; 18% BD, Table 1). Scores on clinician-rated (MADRS) and self-report (BDI-II) depression rating scales reflected “severe” depression (Table 1).
| Variable | Labels | All (n = 97) | AT (n = 47) | TAU (n = 50) |
|---|---|---|---|---|
| Age (years) | Mean (SD) | 36.4 (13.9) | 39.4 (13.8) | 33.7 (13.6) |
| Gender | Female n (%) | 53 (54.6) | 25 (53.2) | 28 (56.0) |
| Male n (%) | 44 (45.4) | 22 (46.8) | 22 (44.0) | |
| Estimated verbal IQ (NART) | Mean (SD) | 108.1 (7.3) | 109.4 (7.6) | 106.9 (7.0) |
| Education (years) | Secondary, mean (SD) | 4.3 (1.4) | 4.1 (1.7) | 4.4 (1.0) |
| Tertiary, mean (SD) | 1.9 (2.0) | 2.1 (2.0) | 1.7 (1.9) | |
| Employment | Employed n (%) | 48 (50.0) | 23 (50.0) | 25 (50.0) |
| Unemployed n (%) | 48 (50.0) | 23 (50.0) | 25 (50.0) | |
| Ethnicity | NZ European n (%) | 60 (61.9) | 28 (59.6) | 32 (64.0) |
| Māori n (%) | 21 (21.6) | 9 (19.1) | 12 (24.0) | |
| Other n (%) | 16 (16.5) | 10 (21.3) | 6 (12.0) | |
| Primary diagnosis | MDD n (%) | 79 (81.4) | 38 (80.9) | 41 (82.0) |
| BD n (%) | 18 (18.6) | 9 (19.1) | 9 (18.0) | |
| Depression symptoms, MADRS | Median (IQR) | 37.0 (32.0–42.0) | 38.0 (35.0–43.0) | 35.5 (30.0–42.0) |
| Depression symptoms, BDI-II | Median (IQR) | 43.0 (33.0–49.0) | 44.0 (33.0–50.0) | 40.5 (30.0–47.8) |
| Illness duration (years) | Median (IQR) | 13.0 (7.0–24.5) | 15.5 (8.0–25.8) | 11.0 (5.0–20.0) |
| Mood disorder onset (age in years) | Median (IQR) | 16.0 (12.5–22.5) | 17.0 (13.0–25.0) | 15.0 (12.0–20.0) |
| Single or recurrent depression | Single n (%) | 13 (13.4) | 4 (8.5) | 9 (18.0) |
| Recurrent n (%) | 84 (86.6) | 43 (91.5) | 41 (82.0) | |
| Psychotic symptoms | Yes n (%) | 34 (35.1) | 20 (42.6) | 14 (28.0) |
| No n (%) | 63 (64.9) | 27 (57.4) | 36 (72.0) | |
| Medication type | Anticonvulsants n (%) | 14 (14.4) | 7 (14.9) | 7 (14.0) |
| Antidepressants n (%) | 85 (87.6) | 43 (91.5) | 42 (84.0) | |
| Antipsychotics n (%) | 59 (60.8) | 31 (66.0) | 28 (56.0) | |
| Anxiolytics n (%) | 75 (77.3) | 36 (76.6) | 39 (78.0) | |
| Lithium n (%) | 8 (8.2) | 5 (10.6) | 3 (6.0) | |
| ECTa | Yes n (%) | 7 (20.0) | 4 (23.5) | 3 (16.7) |
| No n (%) | 28 (80.0) | 13 (76.5) | 15 (83.3) |
- Abbreviations: BD, bipolar disorder; BDI-II, Beck Depression Inventory, 2nd version; ECT, electroconvulsive therapy; IQR, interquartile range; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; NART, National Adult Reading Test; SD, standard deviation.
- a The number of patients who received ECT following the change in protocol (i.e., removal of ECT as an exclusion), which occurred after 62 patients were recruited into the study. Therefore, percentages calculated based on a possible 35 (AT, n = 17, TAU, n = 18) patients.
Given the difference in age between treatment arms (AT = 39.4 years [13.8], TAU = 33.7 years [13.6]), sensitivity analyses for primary and secondary outcomes included age as a covariate. Baseline performance on each secondary outcome was also included in sensitivity analyses.
3.3 Treatment Adherence and Satisfaction
Full details of AT treatment dose are presented in Data S4. The mean number of individual AT sessions in the full sample (n = 97) was 7.3 (SD 1.8) out of a maximum of 8 sessions, the number of minutes in AT sessions was 322.4 (SD 129.1) or 5.4 h, and the number of minutes practicing computerised exercises was 182.1 (SD 178.3) or 3.0 h.
Thirty patients (63.8%) completed the AT treatment ‘per-protocol’ (≥ 5 AT sessions and ≥ 90 min of cognitive exercises). For the per-protocol sample, the mean number of AT sessions was 7.9 (SD 0.6), total minutes in AT sessions was 346.7 (SD 115.9) or 5.8 h, and total minutes spent practicing cognitive exercises was 250.8 (SD 179.2) or 4.2 h.
Seventy percent of patients in the AT arm were satisfied with the content of sessions, and 91% were satisfied with the therapeutic relationship (rated ≥ 5 out of 7). In rating the cognitive activation component, 76% indicated that they were satisfied (rated 4 or 5 out of 5).
Medication status of patients at baseline and 14 weeks is presented in Data S5.
3.4 Primary Outcome
Regression analysis showed that the rate of readmission to psychiatric hospital within 12-weeks following discharge was not significantly different between groups (34% in the AT arm [16 of 47 patients] versus 40% [20 of 50 patients] in the TAU arm) for the ITT analysis (OR = 0.76, CI = 0.30–1.90, p = 0.55), the per protocol analysis (AT, n = 30; TAU, n = 48; OR = 1.26, CI = 0.43–3.65, p = 0.67) or a sensitivity analysis adding age as a covariate in the per protocol sample (OR = 1.32, CI = 0.45–3.88, p = 0.61).
3.5 Secondary Outcomes
Secondary outcomes are presented in Table 2 (ITT analysis) and in Table 3 (per protocol and sensitivity analyses).
| AT | TAU | F b | P b | d c | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Objective cognitive functioning | |||||||
| Global functioning z-score | 0.08 | 0.51 | −0.07 | 0.52 | 2.14 | 0.14 | 0.30 |
| Executive functioning and attention z-score | 0.09 | 0.44 | −0.08 | 0.59 | 2.45 | 0.12 | 0.32 |
| Verbal learning and memory z-score | 0.18 | 0.78 | −0.16 | 0.82 | 4.30 | 0.04 | 0.42 |
| Visuospatial learning and memory z-score | −0.09 | 0.83 | 0.08 | 0.97 | 0.87 | 0.35 | 0.19 |
| Psychomotor speed z-score | 0.15 | 0.79 | −0.13 | 0.65 | 3.71 | 0.05 | 0.40 |
| Depression measures | |||||||
| MADRS total score | 17.36 | 13.44 | 13.54 | 11.92 | 2.27 | 0.14 | 0.31 |
| BDI-II total score | 11.21 | 13.26 | 11.00 | 13.71 | 0.01 | 0.93 | 0.02 |
| Subjective cognitive functioning | |||||||
| COBRA total score | 3.76 | 7.31 | 2.76 | 5.67 | 0.59 | 0.44 | 0.16 |
| Functioning measures | |||||||
| FAST total score | 13.40 | 16.09 | 5.36 | 11.19 | 8.22 | 0.005 | 0.58 |
| QOL.BD total score | 7.49 | 9.80 | 5.78 | 9.35 | 0.81 | 0.37 | 0.18 |
| Activation measures | |||||||
| BADS total score | 7.66 | 10.00 | 5.32 | 11.30 | 1.15 | 0.28 | 0.22 |
| Activation composite score | 7.04 | 7.00 | 5.44 | 7.30 | 1.21 | 0.27 | 0.22 |
- Abbreviations: AT, activation therapy; BADS, Behavioural Activation for Depression Scale–Short Form; BDI-II, Beck Depression Inventory-2nd version; COBRA, Cognitive Complaints in Bipolar Disorder Rating Assessment; FAST, Functioning Assessment Short Test; MADRS, Montgomery–Åsberg Depression Rating Scale; QoL.BD, Quality of Life in Bipolar Disorder; TAU, Treatment-as-usual.
- a Change scores are calculated so that a positive change score reflects improvement from baseline.
- b Univariate ANCOVAs, df (1, 93) with stratum as covariate: study site and mood disorder diagnosis (MDD, BD).
- c Effect size, equivalent to Cohen's d.
| AT | TAU | Main Analysisb | Sensitivity Analysisc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | P | d d | F | P | d d | |
| Objective cognitive functioning | ||||||||||
| Global functioning z-score | 0.20 | 0.65 | −0.09 | 0.61 | 2.94 | 0.09 | 0.45 | 3.61 | 0.06 | 0.50 |
| Executive functioning and attention z-score | 0.21 | 0.54 | −0.08 | 0.70 | 2.93 | 0.09 | 0.45 | 3.30 | 0.07 | 0.49 |
| Verbal learning and memory z-score | 0.31 | 1.00 | −0.24 | 0.95 | 4.66 | 0.03 | 0.56 | 6.83 | 0.01 | 0.70 |
| Visuospatial learning and memory z-score | −0.16 | 1.07 | 0.11 | 1.13 | 0.04 | 0.36 | 0.24 | 0.30 | 0.59 | 0.19 |
| Psychomotor speed z-score | 0.44 | 0.99 | −0.11 | 0.76 | 5.97 | 0.02 | 0.64 | 4.41 | 0.04 | 0.58 |
| Depression measures | ||||||||||
| MADRS total score | 22.91 | 11.01 | 16.46 | 11.71 | 4.52 | 0.04 | 0.56 | 3.00 | 0.08 | 0.46 |
| BDI-II total score | 17.04 | 14.36 | 14.81 | 14.08 | 0.33 | 0.56 | 0.15 | 0.03 | 0.87 | 0.05 |
| Subjective cognitive functioning | ||||||||||
| COBRA total score | 5.50 | 9.45 | 3.72 | 6.33 | 0.72 | 0.39 | 0.22 | 0.66 | 0.42 | 0.22 |
| Functioning measures | ||||||||||
| FAST total score | 16.91 | 15.67 | 6.35 | 12.55 | 8.29 | 0.006 | 0.75 | 3.69 | 0.06 | 0.53 |
| QOL.BD total score | 10.71 | 11.05 | 7.81 | 10.13 | 1.05 | 0.31 | 0.27 | 0.11 | 0.74 | 0.09 |
| Activation measures | ||||||||||
| BADS total score | 11.29 | 10.48 | 7.18 | 12.65 | 1.75 | 0.19 | 0.35 | 0.08 | 0.78 | 0.08 |
| Activation composite score | 10.29 | 6.29 | 7.19 | 7.72 | 2.64 | 0.11 | 0.43 | 1.14 | 0.29 | 0.29 |
- Abbreviations: AT, activation therapy; BADS, Behavioural Activation for Depression Scale; BDI-II, Beck Depression Inventory–2nd version; COBRA, Cognitive Complaints in Bipolar Disorder Rating Assessment; FAST, Functioning Assessment Short Test; MADRS, Montgomery–Åsberg Depression Rating Scale; QoL.BD, Quality of Life in Bipolar Disorder; TAU, Treatment as usual.
- a Change scores were calculated so that a positive change score reflects an improvement from baseline.
- b Univariate ANCOVAs in per protocol sample, df (1, 58), with strata as covariates: study site and mood disorder diagnoses (MDD, BD).
- c Univariate ANCOVAs in the per protocol sample, df (1, 58), with strata (study site, mood disorder diagnosis) and additional covariates: (1) age, (2) baseline score in the respective variable.
- d Effect size, equivalent to Cohen's d.
3.5.1 Objective Cognitive Functioning
For ITT analysis, the AT treatment arm improved significantly more than the TAU arm on verbal learning and memory (F1,93 = 4.30, p = 0.04, d = 0.42) between baseline and 14-week follow-up (Table 2). A trend was found in the same direction for psychomotor speed (F1,93 = 3.71, p = 0.05, d = 0.40, Table 2). For per protocol analysis, these effects were larger, with both domains improving significantly more in AT versus TAU arms (verbal learning and memory: F1,58 = 4.66, p = 0.03, d = 0.56; psychomotor speed: F1,58 = 5.97, p = 0.02, d = 0.64; Table 3). In sensitivity analyses (adding age and baseline performance on the respective measure as covariates), significant improvement in AT versus TAU arms remained for these two cognitive domains (verbal learning and memory: F1,58 = 6.83, p = 0.01, d = 0.70; psychomotor speed: F1,58 = 4.41, p = 0.04, d = 0.58; Table 3). There was no significant difference between treatment arms for the remaining cognitive domains.
3.5.2 Other Secondary Measures
Self-reported general functioning (FAST) improved significantly more in the AT versus TAU treatment arm (F1,93 = 8.22, p = 0.005, d = 0.58, Table 2). Per-protocol analysis of FAST scores showed a greater effect of treatment (F1,58 = 8.29, p = 0.006, d = 0.75, Table 3). However, when controlling for age and baseline FAST score (sensitivity analysis), this effect reduced to a trend (F1,58 = 3.69, p = 0.06, d = 0.53, Table 3).
No significant differences over time between AT and TAU treatment arms were found for any other secondary outcomes (MADRS, BDI-II, COBRA, QoL.BD, BADS, Activation Composite) in ITT analyses (Table 2).
Per protocol, analysis showed a significant effect for clinician-rated mood (MADRS). Mood ratings on the MADRS improved significantly more in the AT treatment arm compared with the TAU arm over time (F1,58 = 4.52, p = 0.04, d = 0.56, Table 3). When baseline MADRS score and age were controlled for (sensitivity analysis), this effect reduced to a trend level (F1,58 = 3.00, p = 0.08, d = 0.46, Table 3).
On the remaining secondary outcome measures (BDI-II, COBRA, QoL.BD, BADS, Activation Composite), no significant differences over time between the AT and TAU treatment arms were found in per-protocol analyses (Table 3).
3.6 Dose of Activation Therapy
Pearson's correlation analyses were conducted to evaluate the association between secondary outcome measures and AT dose. No significant correlations between the number of minutes spent on cognitive exercises and the change in any of the secondary outcome measures were found (all p > 0.05).
3.7 Adverse Events
There was one self-reported suicide attempt in the AT treatment arm. This was not deemed by the individual to be related to the study interventions. No further serious adverse events were reported.
4 Discussion
In this pragmatic RCT, a 2-week course of AT, compared with TAU, did not reduce readmission rates to psychiatric hospitals in the 12 weeks following discharge. Verbal learning and memory, and self-reported general functioning improved significantly more in AT versus TAU arms over 14 weeks. Significant effects in per-protocol and sensitivity analyses are discussed below.
4.1 No Difference in Readmission Rates With Activation Therapy
Rate of readmission has been proposed as a useful indicator of the quality of previous inpatient psychiatric care [30]. In New Zealand, inpatient care of an MDE focuses on short-term stabilization of the current mood episode with medication [31]. Inpatient stays last an average of 2–3 weeks. International surveys consistently report concerns regarding over-reliance on medication and failure to provide therapeutic environments during inpatient admissions [32]. Rates of readmission following discharge are unsurprisingly high—in Canterbury, New Zealand, from 2011 to 2016, 40% of individuals initially hospitalized for an MDE were readmitted within 12 weeks of discharge. Activation Therapy was therefore designed as an intensive psychological therapy that could be delivered during an inpatient stay, targeting key features of inpatient depression (‘deactivation’ and cognitive impairment), aiming to reduce readmission post-discharge. However, readmission rates were not significantly reduced, being similar to existing rates for both arms (34% AT, 40% TAU). This was the case also for the sub-group who engaged in and received an adequate dose of AT. This negative finding may relate to the short-term course of AT, with no follow-up or maintenance sessions after the 2-week treatment period. Patients admitted to hospital with MDE in New Zealand usually present with severe mood symptomatology and significant psychosocial stress. Ongoing psychosocial stressors in the community may have prevented the durability of treatment gains.
4.2 Improved Aspects of Cognition and Functioning With Activation Therapy
The strongest signal of improvement with AT in the current trial was for measures of objective cognitive function. Verbal learning and memory improved significantly more following AT than following TAU, with a small effect size difference (d = 0.42). Psychomotor speed also improved with a similar small effect (d = 0.40) but only at trend level in the ITT analysis. When examining inpatients who received an adequate dose of AT (per protocol), these effects in both domains increased to being moderate (d = 0.56–0.64) and statistically significant.
These findings are broadly consistent with recent meta-analyses of ‘in-episode’ depression samples, which have found small to moderate effects of CR across cognitive domains, except for visuospatial memory [33, 34]. Only two studies have investigated computerised cognitive training in inpatient samples; Trapp et al. [35] conducted a pilot RCT (n = 46) comparing CR (12 sessions over 4 weeks) with TAU, and Semkovska et al. [36] conducted a feasibility and pilot trial (n = 24) comparing CR (20 sessions over 5 weeks) with an active control condition. Both studies reported improved aspects of cognitive function at treatment end, particularly in memory, executive function, and working memory.
Pro-cognitive effects in this trial are noteworthy for a number of reasons. First, AT was shorter in duration and involved fewer sessions than the previous inpatient studies [35, 36]. Second, the follow-up cognitive assessment occurred 12 weeks after the end of the AT treatment period, representing the durability of cognitive change. In contrast, Trapp et al. [35] and Semkovka et al.'s [36] cognitive assessments occurred at treatmentend. Third, we found a significant improvement in our measure of general functioning (FAST) with a moderate effect (d = 0.58). Scores on the FAST improved significantly more in the AT versus TAU arms in per protocol analyses too (d = 0.75), but when controlling for baseline FAST score and age, this effect was reduced to a trend (d = 0.53). The data is of importance given a lack of data from RCTs on the impact of CR on longer-term cognition and functioning in ‘in-episode’ MDE trials [34], with most trials assessing short-term improvement in depression scores and cognition.
4.3 Was ‘De-Activation’ Adequately Targeted With Activation Therapy?
There was no strong signal of improvement in depression symptoms or activation measures with AT in this trial. Our two measures of behavioural activation (BADS, activation composite) did not show evidence of significantly greater improvement with AT. This is notable given that these measures assess what was specifically targeted in BA (e.g., engagement and enjoyment in activities).
There are several considerations when interpreting these findings in the context of previous BA and CR studies. In relation to most previous BA studies, the current AT therapy was intensive but brief. Meta-analysis has reported large effect size differences between BA and control conditions in depression scores over treatment [37], with larger effects for more severely depressed samples (moderate to severe depression). In comparison with the current study, studies included in Ekers et al.'s meta-analysis [37] delivered a similar number of BA sessions (median number = 8) but over a longer treatment period (usually 6–12 weeks). A longer period may allow for greater engagement in BA and more time to make enduring changes in behaviours and day-to-day routines. Further, we note again that there were 12 weeks between AT ending and the 14-week follow-up assessment, when depression measures reported here were conducted.
Another consideration is whether patients in this study engaged with therapy, especially in the context of very severe depression. Sixty-four percent of patients completed the pre-defined ‘adequate dose’ of therapy. Very few BA trials have been conducted in inpatient settings; however, broadly similar findings have been reported in BA trials of less severely depressed outpatient and community samples [38, 39]. The large (n = 221), UK-based COBRA trial [39] reported that 67% of their BA sample received their pre-defined ‘sufficient dose’ of therapy (≥ 8 out of 20 sessions). These results do suggest that BA is feasible, even in very severely depressed (mean MADRS = 38) patients.
Activation Therapy was designed so that the two components (BA and cognitive activation) could be complementary, for example, scheduling cognitive exercises into daily activity scheduling forms. However, in combining two therapeutic frameworks, there is the risk of adding complexity and potentially diluting treatment-related gains. In our previous RCT comparing Interpersonal and Social Rhythm Therapy (IPSRT) with IPSRT and CR, the addition of CR did appear to dilute the effects of IPSRT on functioning and mood outcomes [29].
5 Limitations
Specific issues in this trial in explaining negative findings relating to readmission rates (short course of AT with no maintenance sessions) and mood symptoms (primary follow-up time-point occurring 12 weeks after treatment-end, potential dilution of therapies by using two therapeutic frameworks) have been discussed in previous sections of this discussion. The most notable limitation to this study as a whole, however, is that the trial did not reach its recruitment target (n = 165) and was therefore underpowered. This was due to continued shortages in research staff at secondary sites and the pressure of the COVID pandemic. Second, we did not enrich the trial for cognitive impairment. The ISBD Targeting Cognition Taskforce recommends screening for objective and subjective cognitive impairment in clinical trials of cognitive interventions [23]. We opted not to screen for cognitive impairment in this trial due to our focus on being able to translate interventions to clinical practice. Screening with objective cognitive testing is less feasible in a busy inpatient setting. Third, related to the pragmatic nature of this trial, we recruited individuals across the mood disorder spectrum. While this can be seen as a strength of the study from a translatability perspective, having a sample with varied mood disorder diagnoses may obscure more specific effects that AT has on particular mood disorder diagnoses.
6 Conclusions
This study contributes to a growing body of literature examining the effectiveness of BA and Cognitive Activation for depression, and extends these combined modalities to an inpatient population. The AT intervention in this RCT was feasible in severely depressed inpatients, but it did not statistically significantly improve readmission rates. Aspects of cognitive function showed significant improvement in the AT versus TAU arm, 12-weeks after treatment-end. Future analyses of this data will examine predictors of cognitive and mood improvement, as well as a better understanding of the patient experience of AT using qualitative analysis of post-treatment interviews. As this study was underpowered, mainly due to COVID-related disruptions, further larger trials are warranted.
Author Contributions
Richard J. Porter was the principal investigator, and Richard J. Porter and Katie M. Douglas were involved in all aspects of study conception and design. Jennifer Jordan, Marie T. Crowe, Cameron J. Lacey, Cecilia Smith Hamel, Ian R. E. Averill, and Christopher R. Bowie were study co-investigators and contributed to study design. Cameron J. Lacey provided Māori health advice and supervision. Jennifer Jordan, Katie M. Douglas, and Christopher R. Bowie provided expertise on the design of AT and training of AT. Christopher M. Frampton and Richard J. Porter provided statistical expertise, and Katie M. Douglas and Zoe A. Odering completed statistical analyses. Katie M. Douglas, Zoe A. Odering, and Richard J. Porter drafted this paper, and all authors have edited and critically reviewed the paper for intellectual content and approved the final version.
Acknowledgements
The authors would like to acknowledge the crucial roles of Bridget Kimber, Rachel Day-Brown, Aroha De Bie, Robin Farmar, Emma McKenzie, Chrissie Muirhead, Gabrielle Nolan, Mere Porima, Hayley Wells, Lysandra Low, Tania Maguigan (research nurses), Emily Douglas (research assistant), and Andrea Bartram (data manager) in this study. The authors would also like to acknowledge the ward staff at Te Ao Marama and Te Awakura Acute Inpatient Service for their support in the running of this study. Open access publishing facilitated by University of Otago, as part of the Wiley - University of Otago agreement via the Council of Australian University Librarians.
Conflicts of Interest
K.D., C.B., and R.P. use software provided free-of-charge by Scientific Brain Training Pro for Cognitive Remediation trials. R.P. has received support for travel to educational meetings from Servier and Lundbeck. CB has grant support from Lundbeck, Takeda, and Pfizer and has received consulting fees from Boehringer Ingelheim, Pfizer, and Lundbeck. All other authors have no competing interests to declare.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, KD, upon reasonable request.




