TCA Cycle Intermediate Mitigates Di(2-ethylhexyl) Phthalate-Induced Cholestatic Liver Injury Through Modulation of the Nrf2/NQO1 Signalling Axis
Yue Jiang
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorFang Xie
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorXutao Ling
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorJiayi Zhang
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorYun Yu
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
Search for more papers by this authorQianqian Huang
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
Search for more papers by this authorLun Zhang
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
Search for more papers by this authorLu Ye
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorWenkang Tao
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorMengzhen Hou
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorCorresponding Author
Cheng Zhang
Department of Toxicology, Anhui Medical University, Hefei, China
Key Laboratory of Environmental Toxicology of Anhui Higher Education Institutes, Anhui Medical University, Hefei, China
MOE Key Laboratory of Population Health Across Life Cycle, Hefei, China
Correspondence:
Cheng Zhang ([email protected])
Jianqing Wang ([email protected])
Search for more papers by this authorCorresponding Author
Jianqing Wang
School of Pharmacy, Anhui Medical University, Hefei, China
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
MOE Key Laboratory of Population Health Across Life Cycle, Hefei, China
Correspondence:
Cheng Zhang ([email protected])
Jianqing Wang ([email protected])
Search for more papers by this authorYue Jiang
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorFang Xie
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorXutao Ling
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorJiayi Zhang
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorYun Yu
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
Search for more papers by this authorQianqian Huang
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
Search for more papers by this authorLun Zhang
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
Search for more papers by this authorLu Ye
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorWenkang Tao
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorMengzhen Hou
School of Pharmacy, Anhui Medical University, Hefei, China
Search for more papers by this authorCorresponding Author
Cheng Zhang
Department of Toxicology, Anhui Medical University, Hefei, China
Key Laboratory of Environmental Toxicology of Anhui Higher Education Institutes, Anhui Medical University, Hefei, China
MOE Key Laboratory of Population Health Across Life Cycle, Hefei, China
Correspondence:
Cheng Zhang ([email protected])
Jianqing Wang ([email protected])
Search for more papers by this authorCorresponding Author
Jianqing Wang
School of Pharmacy, Anhui Medical University, Hefei, China
Department of Pharmacy, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Anhui Public Health Clinical Center, Hefei, China
MOE Key Laboratory of Population Health Across Life Cycle, Hefei, China
Correspondence:
Cheng Zhang ([email protected])
Jianqing Wang ([email protected])
Search for more papers by this authorFunding: This project was supported by the National Natural Science Foundation of China (No. 82073566), the University Young and Middle-aged Teacher Training Project from the Educational Commission of Anhui Province (DTR2023012), the Health Research Project of Anhui Province (AHWJ2023A20294), the Anhui Public Health Clinical Center, the First Affiliated Hospital of Anhui Medical University North Area Scientific Research Cultivation Fund Project (2023YKJ11, 2023YKJ06 and 2023YKJ14) and the Anhui Medical University Scientific Research Fund project (2023xkj043).
ABSTRACT
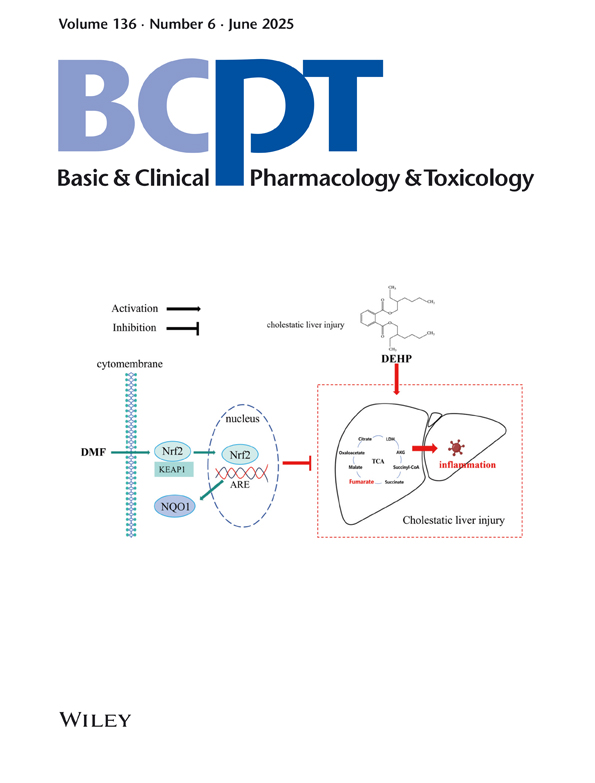
As a commonly used phthalate compound, di(2-ethylhexyl) phthalate (DEHP) has been shown to disrupt the tricarboxylic acid (TCA) cycle and aggravate tissue damage. However, whether the TCA cycle is involved in cholestatic liver injury (CLI) induced by DEHP and the protective effect of dimethyl fumarate (DMF), which is used to supplement TCA intermediate metabolites, remained unclear. Here, mice were randomized into five groups (n = 6/group): (1) Control, (2) DEHP (200 mg/kg/day), (3) DMF (100 mg/kg/day), (4) DEHP + DMF (30 mg/kg/day) and (5) DEHP + DMF (100 mg/kg/day). Our data demonstrated that DEHP exposure upregulated total bile acid (TBA) levels and broke the TCA cycle, resulting in reduced fumaric acid and malic acid. However, we further supplemented fumaric acid with DMF and found that DMF effectively reversed the high levels of TBA, alkaline phosphatase (ALP) and glutamyl transpeptidase (GGT) induced by DEHP in mice. Meanwhile, pathological results in the liver showed that DMF improved bile duct cell damage, inflammatory cell infiltration, collagen deposition and necrosis caused by DEHP. In addition, we found that DEHP elevated the level of interleukin (IL)-1β, IL-6, TNF-α and MDA and decreased the level of SOD in the mouse liver, which was effectively reversed by DMF treatment. Besides, DMF upregulated the expression of Nrf2 and NQO1 in the liver of DEHP-exposed mice. For in vitro validation, AML-12 cells were treated with (1) Control, (2) DEHP (250 μM), (3) DEHP + DMF (10 μM), (4) DEHP + DMF (25 μM) and (5) DEHP + DMF (50 μM). DEHP exposure increased the expression of IL-1β, IL-6 and TNF-α, which was mitigated by DMF, while ML385, an Nrf2 inhibitor, could counteract the anti-inflammatory effects of DMF. These findings indicate that DEHP broke the TCA cycle of the mouse liver, and DMF supplementation protects against DEHP-induced CLI by activating the Nrf2/NQO1 pathway.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1Y. Zhao, H. X. Li, Y. Luo, J. G. Cui, M. Talukder, and J. L. Li, “Lycopene Mitigates DEHP-Induced Hepatic Mitochondrial Quality Control Disorder via Regulating SIRT1/PINK1/Mitophagy Axis and Mitochondrial Unfolded Protein Response,” Environmental Pollution 292 no. Pt B (2022): 118390.
10.1016/j.envpol.2021.118390 Google Scholar
- 2Y. Q. Huang, Y. X. Tang, B. H. Qiu, M. Talukder, X. N. Li, and J. L. Li, “Di-2-Ethylhexyl Phthalate (DEHP) Induced Lipid Metabolism Disorder in Liver via Activating the LXR/SREBP-1c/PPARalpha/Gamma and NF-kappaB Signaling Pathway,” Food and Chemical Toxicology 165 (2022): 113119.
- 3D. Lo, Y. T. Wang, and M. C. Wu, “Hepatoprotective Effect of Silymarin on Di(2-ethylhexyl)phthalate (DEHP) Induced Injury in Liver FL83B Cells,” Environmental Toxicology and Pharmacology 38, no. 1 (2014): 112–118.
- 4Y. Zhang, H. Qian, J. Wang, et al., “Di-(2-ethylhexyl) Phthalate (DEHP) Promoted Hepatic Lipid Accumulation by Activating Notch Signaling Pathway,” Environmental Toxicology 38, no. 7 (2023): 1628–1640.
- 5J. C. Caldwell, “DEHP: Genotoxicity and Potential Carcinogenic Mechanisms—A Review,” Mutation Research 751, no. 2 (2012): 82–157.
- 6Z. Yu, Z. Shi, Z. Zheng, et al., “DEHP Induce Cholesterol Imbalance via Disturbing Bile Acid Metabolism by Altering the Composition of Gut Microbiota in Rats,” Chemosphere 263 (2021): 127959.
- 7Q. Shi, X. Yuan, Y. Zeng, et al., “Crosstalk Between Gut Microbiota and Bile Acids in Cholestatic Liver Disease,” Nutrients 15, no. 10 (2023): 15102411.
- 8S. Y. Cai and J. L. Boyer, “The Role of Bile Acids in Cholestatic Liver Injury,” Annals of Translational Medicine 9, no. 8 (2021): 737.
- 9M. Li, S. Y. Cai, and J. L. Boyer, “Mechanisms of Bile Acid Mediated Inflammation in the Liver,” Molecular Aspects of Medicine 56 (2017): 45–53.
- 10A. A. M. El-Faramawy, L. B. E. El-Shazly, A. A. Abbass, et al., “Serum IL-6 and IL-8 in Infants With Biliary Atresia in Comparison to Intrahepatic Cholestasis,” Tropical Gastroenterology 32, no. 1 (2011): 50–55.
- 11M. Sasaki, M. Miyakoshi, Y. Sato, et al., “Chemokine-Chemokine Receptor CCL2-CCR2 and CX3CL1-CX3CR1 Axis May Play a Role in the Aggravated Inflammation in Primary Biliary Cirrhosis,” Digestive Diseases and Sciences 59, no. 2 (2014): 358–364.
- 12Z. Weng, Y. Chi, J. Xie, et al., “Anti-Inflammatory Activity of Dehydroandrographolide by TLR4/NF-kappaB Signaling Pathway Inhibition in Bile Duct-Ligated Mice,” Cellular Physiology and Biochemistry 49, no. 3 (2018): 1083–1096.
- 13X. Luan, P. Chen, Y. Li, et al., “TNF-Alpha/IL-1beta-Licensed hADSCs Alleviate Cholestatic Liver Injury and Fibrosis in Mice via COX-2/PGE2 Pathway,” Stem Cell Research & Therapy 14, no. 1 (2023): 100.
- 14R. Heidari and H. Niknahad, “The Role and Study of Mitochondrial Impairment and Oxidative Stress in Cholestasis,” Methods in Molecular Biology 2019 (1981): 117–132.
- 15Q. Gao, G. Li, Y. Zu, et al., “Ginsenoside Rg1 Alleviates ANIT-Induced Cholestatic Liver Injury by Inhibiting Hepatic Inflammation and Oxidative Stress via SIRT1 Activation,” Journal of Ethnopharmacology 319, no. Pt 1 (2024): 117089.
- 16E. J. Lee, Y. P. Hong, and Y. J. Yang, “Short-Term Exposure to Di(2-ethylhexyl)phthalate May Disrupt Hepatic Lipid Metabolism Through Modulating the Oxidative Stress in Male Adolescent Rats,” Environmental Analysis Health and Toxicology 39, no. 1 (2024): e2024007–0.
- 17Z. B. Zhao, K. Ji, X. Y. Shen, et al., “Di(2-ethylhexyl) Phthalate Promotes Hepatic Fibrosis by Regulation of Oxidative Stress and Inflammation Responses in Rats,” Environmental Toxicology and Pharmacology 68 (2019): 109–119.
- 18P. H. Liao, H. H. Hsu, T. S. Chen, et al., “Phosphorylation of Cofilin-1 by ERK Confers HDAC Inhibitor Resistance in Hepatocellular Carcinoma Cells via Decreased ROS-Mediated Mitochondria Injury,” Oncogene 36, no. 14 (2017): 1978–1990.
- 19R. Zhao, Q. Zhao, X. Wang, et al., “Yi-Qi-Jian-Pi Formula Inhibits Hepatocyte Pyroptosis Through the IDH2-Driven Tricarboxylic Acid Cycle to Reduce Liver Injury in Acute-on-Chronic Liver Failure,” Journal of Ethnopharmacology 317 (2023): 116683.
- 20G. Shen, L. Zhou, W. Liu, et al., “Di(2-ethylhexyl)phthalate Alters the Synthesis and Beta-Oxidation of Fatty Acids and Hinders ATP Supply in Mouse Testes via UPLC-Q-Exactive Orbitrap MS-Based Metabonomics Study,” Journal of Agricultural and Food Chemistry 65, no. 24 (2017): 5056–5063.
- 21A. Loboda, M. Damulewicz, E. Pyza, et al., “Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism,” Cellular and Molecular Life Sciences 73, no. 17 (2016): 3221–3247.
- 22E. H. Kobayashi, T. Suzuki, R. Funayama, et al., “Nrf2 Suppresses Macrophage Inflammatory Response by Blocking Proinflammatory Cytokine Transcription,” Nature Communications 7 (2016): 11624.
- 23L. He, C. Guo, C. Peng, and Y. Li, “Advances of Natural Activators for Nrf2 Signaling Pathway on Cholestatic Liver Injury Protection: A Review,” European Journal of Pharmacology 910 (2021): 174447.
- 24M. Sangineto, F. Grabherr, T. E. Adolph, et al., “Dimethyl fumarate Ameliorates Hepatic Inflammation in Alcohol Related Liver Disease,” Liver International 40, no. 7 (2020): 1610–1619.
- 25A. R. Gafson, C. Savva, T. Thorne, et al., “Breaking the Cycle: Reversal of Flux in the Tricarboxylic Acid Cycle by Dimethyl Fumarate,” Neurology Neuroimmunology & Neuroinflammation 6, no. 3 (2019): e562.
- 26D. Olagnier, E. Farahani, J. Thyrsted, et al., “SARS-CoV2-Mediated Suppression of NRF2-Signaling Reveals Potent Antiviral and Anti-Inflammatory Activity of 4-Octyl-Itaconate and Dimethyl Fumarate,” Nature Communications 11, no. 1 (2020): 4938.
- 27N. Yan, Z. Xu, C. Qu, and J. J. Zhang, “Dimethyl Fumarate Improves Cognitive Deficits in Chronic Cerebral Hypoperfusion Rats by Alleviating Inflammation, Oxidative Stress, and Ferroptosis via NRF2/ARE/NF-kappaB Signal Pathway,” International Immunopharmacology 98 (2021): 107844.
- 28M. E. Mostafa, A. A. Shaaban, and H. A. Salem, “Dimethylfumarate Ameliorates Hepatic Injury and Fibrosis Induced by Carbon Tetrachloride,” Chemico-Biological Interactions 302 (2019): 53–60.
- 29P. Tveden-Nyborg, T. K. Bergmann, N. Jessen, U. Simonsen, and J. Lykkesfeldt, “BCPT 2023 Policy for Experimental and Clinical Studies,” Basic & Clinical Pharmacology & Toxicology 133, no. 4 (2023): 391–396.
- 30M. R. Gallop, S. Y. Tobin, and A. Chaix, “Finding Balance: Understanding the Energetics of Time-Restricted Feeding in Mice,” Obesity (Silver Spring) 31, no. 1 (2023): 22–39.
- 31F. Zhao, L. Zhang, M. Qu, et al., “Obeticholic Acid Alleviates Intrauterine Growth Restriction Induced by Di-ethyl-hexyl Phthalate in Pregnant Female Mice by Improving Bile Acid Disorder,” Environmental Science and Pollution Research International 30, no. 51 (2023): 110956–110969.
- 32A. R. Vanani, H. Kalantari, M. Mahdavinia, M. Rashno, L. Khorsandi, and M. J. Khodayar, “Dimethyl Fumarate Reduces Oxidative Stress, Inflammation and Fat Deposition by Modulation of Nrf2, SREBP-1c and NF-kappaB Signaling in HFD Fed Mice,” Life Sciences 283 (2021): 119852.
- 33Z. Xu, W. Tang, Q. Xie, et al., “Dimethyl Fumarate Attenuates Cholestatic Liver Injury by Activating the NRF2 and FXR Pathways and Suppressing NLRP3/GSDMD Signaling in Mice,” Experimental Cell Research 432, no. 2 (2023): 113781.
- 34J. Sampson and D. de Korte, “DEHP-Plasticised PVC: Relevance to Blood Services,” Transfusion Medicine 21, no. 2 (2011): 73–83.
- 35H. Chen, W. Zhang, B. B. Rui, S. M. Yang, W. P. Xu, and W. Wei, “Di(2-ethylhexyl) Phthalate Exacerbates Non-Alcoholic Fatty Liver in Rats and Its Potential Mechanisms,” Environmental Toxicology and Pharmacology 42 (2016): 38–44.
- 36G. Li, C. Y. Zhao, Q. Wu, et al., “Integrated Metabolomics and Transcriptomics Reveal Di(2-ethylhexyl) Phthalate-Induced Mitochondrial Dysfunction and Glucose Metabolism Disorder Through Oxidative Stress in Rat Liver,” Ecotoxicology and Environmental Safety 228 (2021): 112988.
- 37P. H. Pan, Y. Y. Wang, S. Y. Lin, et al., “Plumbagin Ameliorates Bile Duct Ligation-Induced Cholestatic Liver Injury in Rats,” Biomedicine & Pharmacotherapy 151 (2022): 113133.
- 38T. Yang, T. Shu, G. Liu, et al., “Quantitative Profiling of 19 Bile Acids in Rat Plasma, Liver, Bile and Different Intestinal Section Contents to Investigate Bile Acid Homeostasis and the Application of Temporal Variation of Endogenous Bile Acids,” Journal of Steroid Biochemistry and Molecular Biology 172 (2017): 69–78.
- 39J. Y. L. Chiang and J. M. Ferrell, “Bile Acids as Metabolic Regulators and Nutrient Sensors,” Annual Review of Nutrition 39 (2019): 175–200.
- 40M. Singh, A. Singh, S. Kundu, S. Bansal, and A. Bajaj, “Deciphering the Role of Charge, Hydration, and Hydrophobicity for Cytotoxic Activities and Membrane Interactions of Bile Acid Based Facial Amphiphiles,” Biochimica et Biophysica Acta 1828, no. 8 (2013): 1926–1937.
- 41A. P. Rolo, P. J. Oliveira, A. J. Moreno, et al., “Bile Acids Affect Liver Mitochondrial Bioenergetics: Possible Relevance for Cholestasis Therapy,” Toxicological Sciences 57, no. 1 (2000): 177–185.
- 42S. Y. Cai and J. L. Boyer, “The Role of Inflammation in the Mechanisms of Bile Acid-Induced Liver Damage,” Digestive Diseases 35, no. 3 (2017): 232–234.
- 43E. Gonzalez-Sanchez, M. J. Perez, N. S. Nytofte, et al., “Protective Role of Biliverdin Against Bile Acid-Induced Oxidative Stress in Liver Cells,” Free Radical Biology & Medicine 97 (2016): 466–477.
- 44C. T. Shearn, A. L. Anderson, C. G. Miller, et al., “Thioredoxin Reductase 1 Regulates Hepatic Inflammation and Macrophage Activation During Acute Cholestatic Liver Injury,” Hepatology Communications 7, no. 1 (2023): e0020.
- 45W. Q. Ren, N. Liu, Y. Shen, et al., “Subchronic Exposure to Di-(2-ethylhexyl) Phthalate (DEHP) Elicits Blood-Brain Barrier Dysfunction and Neuroinflammation in Male C57BL/6J Mice,” Toxicology 499 (2023): 153650.
- 46X. Zhen, L. Jindong, Z. Yang, et al., “Activation of Nrf2 Pathway by Dimethyl Fumarate Attenuates Renal Ischemia-Reperfusion Injury,” Transplantation Proceedings 53, no. 7 (2021): 2133–2139.
- 47K. Zhao and L.-B. Wen, “DMF Attenuates Cisplatin-Induced Kidney Injury via Activating Nrf2 Signaling Pathway and Inhibiting NF-kB Signaling Pathway,” European Review for Medical and Pharmacological Sciences 22, no. 24 (2018): 8924–8931.
- 48F. He, X. Ru, and T. Wen, “NRF2, a Transcription Factor for Stress Response and Beyond,” International Journal of Molecular Sciences 21, no. 13 (2020): 21134777.
- 49M. Q. Wang, K. H. Zhang, F. L. Liu, et al., “Wedelolactone Alleviates Cholestatic Liver Injury by Regulating FXR-Bile Acid-NF-kappaB/NRF2 Axis to Reduce Bile Acid Accumulation and Its Subsequent Inflammation and Oxidative Stress,” Phytomedicine 122 (2024): 155124.
- 50Y. Zhao, Z. H. Du, M. Talukder, et al., “Crosstalk Between Unfolded Protein Response and Nrf2-Mediated Antioxidant Defense in Di-(2-ethylhexyl) Phthalate-Induced Renal Injury In Quail (Coturnix japonica),” Environmental Pollution 242, no. Pt B (2018): 1871–1879.
- 51T. Toyama, A. P. Looney, B. M. Baker, et al., “Therapeutic Targeting of TAZ and YAP by Dimethyl Fumarate in Systemic Sclerosis Fibrosis,” Journal of Investigative Dermatology 138, no. 1 (2018): 78–88.
- 52W. X. Zhang, J. H. Zhao, F. M. Ping, et al., “Effect of Dimethyl Fumarate on Rats With Chronic Pancreatitis,” Asian Pacific Journal of Tropical Medicine 9, no. 3 (2016): 261–264.
- 53G. O. Gillard, B. Collette, J. Anderson, et al., “DMF, but Not Other Fumarates, Inhibits NF-kappaB Activity In Vitro in an Nrf2-Independent Manner,” Journal of Neuroimmunology 283 (2015): 74–85.
- 54U. Schulze-Topphoff, M. Varrin-Doyer, K. Pekarek, et al., “Dimethyl Fumarate Treatment Induces Adaptive and Innate Immune Modulation Independent of Nrf2,” Proceedings of the National Academy of Sciences of the United States of America 113, no. 17 (2016): 4777–4782.