Population and reproductive structure in the endangered and highly endemic freshwater crab Aegla concepcionensis (Decapoda:Pleocyemata:Aeglidae) from Chile
Abstract
Population and reproductive information of highly endemic species allow us to understand their underlying conservation problems. Aegla concepcionensis is restricted to a small and intervened Chilean basin, and its conservation status has varied from extinct in nature to endangered. We characterized their life history measuring population, morphological and environmental variables during an annual cycle capturing individuals with a catch and release method based on electroshocking. Although the maximum water temperature was related to the recruitment, it can be physiologically risky for the remaining macroinvertebrate community. The relationship between abundance and narrow pH variations indicates a condition of unstable equilibrium given the environmental deterioration by deforestation. Sex ratio was predominantly male biased during copulatory activity, and sexually dimorphic body size distributions supported the hypothesis of greater natural selection in females and sexual selection in males. The period of ovigerous females was comparatively restricted, late and consistent with an efficient ecophysiological strategy of reproductive investment. Patterns of pubertal moult, onset of morphometric maturity, absence of morphological differentiation in both sexes suggested that A. concepcionensis tends to maximize reproductive performance favouring the recovery of their relict populations.
1 INTRODUCTION
South-central Chile is a biodiversity hotspot of priority concern. This is due to the large concentration of endemic species severely threatened by the contamination, transformation and reduction of habitats since the mid-nineteenth century within this area of greater forestry, agricultural and industrial activity in the country (Miranda et al., 2017; Myers et al., 2000). For this reason, strengthening the Chilean system of natural reserves or protected areas has constituted an essential conservation strategy for this biogeographic unit, where the criteria of local valorization have been increasingly relevant to mitigate the loss of biodiversity (Bidegain et al., 2019; Zorondo-Rodríguez et al., 2014). Local conservation initiatives are highly dependent on information on the life history of the species to determine integrated management strategies for taxa and environmentally vulnerable areas (Martín-López et al., 2009). Many populations and reproductive traits have high levels of environmental variance with direct effects on fitness (Stearns, 1992). Therefore, various types of phenotype–environment interactions between population and reproductive traits may emerge as strategies of response to natural selection (Birch, 2016). However, this display of phenotypic expressions may be limited by sources of restriction of different nature, which potentially harm the well-being of the species, and to a great extent may determine their vulnerability to extinction (Jager et al., 2008; Reynolds et al., 2005). This situation is especially relevant in strictly freshwater taxa, where the specificity of habitats, distribution limitations and poor connectivity between populations reduce the probability of gene flow and consequently the capacity for adaptive change in the expression of life-history traits (Pavlova et al., 2017). According to the criteria of the International Union for Conservation of Nature (IUCN), several freshwater taxa with threat of extinction present severely degraded environments and high levels of endemism since their distributions are restricted to exclusive small hydrographic basins (Bland, 2017; Collen et al., 2014). In some cases, the idiosyncratic or charismatic value of taxa threatened by urban growth may promote their prioritization in conservation planning (Gordon et al., 2009) Aegla concepcionensis is a highly endemic species of freshwater anomuran crab restricted to small streams of low order and moderate current velocity in the Andalién River basin in the Coast Range of the Concepción Province (Jara et al., 2006), which includes one of the largest urban and industrial conglomerates in south-central Chile (Aliste et al., 2012). Like about 70% of the 87 species actually described for the genus Aegla (Bartholomei-Santos et al., 2019), A. concepcionensis falls under one of the conservation threat categories established by IUCN, experiencing some variation over time (Bond-Buckup et al., 2008; Santos et al., 2017). The severe alteration of their habitat due to forest exploitation and urbanization caused the absence of reports of this species during the 1980s and 1990s (Jara, 1986, 1992). For this reason, A. concepcionensis was declared Extinct in the Wild in the 2001 Red List Categories report (Pérez-Losada et al., 2002). However, in the mid-2000s this species was rediscovered in a relictual stream (Estero Cárcamo) within the main campus of the University of Concepción (Valdovinos, 2006, 2008). Subsequent prospecting efforts increased the number of records in small streams associated with the Andalién River basin located on the southern outskirts of Concepción city (Iraira et al., 2018). For this, the species was reassigned to the Critically Endangered category in the 2007 and 2012 reports of the Red List Categories (Pérez-Losada et al., 2009; Santos et al., 2017). The current greater frequency and intensity of summer forest fires constitute an emerging threat to the distribution area of A. concepcionensis (Urrutia-Jalabert et al., 2018). However, its categorization as Endangered species by the Chilean Government through Decreto Supremo Nº52/2014 together with scientific and citizen efforts tending to grant patrimonial value to the forest habitat and particularly to A. concepcionensis has promoted important management and ecosystem conservation efforts. For this, the scientific community has indicated that it is essential to know the population ecological aspects associated with the habitat, feeding and reproductive requirements of A. concepcionensis (Jara, 2005; Jara et al., 2006).
The environmental conditions in a fluvial relict inhabited by A. concepcionensis were measured monthly. In parallel, the application of a representative, without mortality, and based on catch and release sampling design allowed to measure in situ some phenotypic attributes and population of this species. Thus, the temporal fluctuation of the demographic and reproductive composition of A. concepcionensis and its phenotype–environment relationships were analysed during an annual cycle. Due to the marked environmental seasonality typically expected in streams of South-Central Chile (Correa-Araneda & Salazar, 2014), we propose a significant environmental influence on the demographic and reproductive deployment of A. concepcionensis with relevant adaptive implications. The information derived from this study will be relevant to the population biological knowledge of this species due to its state of vulnerability and consequently for the adoption of future conservation measures.
2 MATERIALS AND METHODS
We studied a micro-basin tributary of the main channel of the Andalién River, which is located in the Coast Range in south-central Chile. Nine sampling points were located in a section 1.7 km long in the tributary streams of the Chaimávida sub-basin, a low-order tributary stream present in the southeast section of the Andalién River (Figure 1). Previous sampling performed by the first author suggests that these areas have a particular abundance of A. concepcionensis within the Andalién River basin.
Between July, 2018 and June, 2019, the sampling points were monthly measured in terms of the structure of the population abundance of A. concepcionensis and the environmental characteristics of the water column. Individuals of A. concepcionensis were obtained using a low-intensity electric fishing protocol previously implemented in catch and release procedures (Catchpole & Ruiz, 2018). At each sampling point, diurnal sampling was performed with a Samus 725MP portable electric fishing equipment at low frequency (<30 Hz) and an output power <100 W. Exposure to this electrical condition reduces both appendage shedding and damage by galvanonarcosis (Catchpole & Ruiz, 2018; Taylor et al., 2001). A fishing period of 40 min was established to standardize the sampling effort, and the lethargic individuals were captured with a D-frame net (0.25 m2 aperture and 0.5 mm frame opening) and deposited in plastic containers kept at water temperature. The environmental characterization was carried out by measuring the physical–chemical variability with a previously calibrated Hanna HI9829 multiparameter equipment that recorded the temperature (°C), pH (pH units), redox potential (mV), dissolved oxygen (mg*L−1), electrical conductivity (μs*cm−1) and total dissolved solids (mg*L−1) in the water column. This measurement was carried out at some randomly selected sampling points. Therefore, the representation of each parameter was represented by its variability with respect to a seasonal mean.
The sex of the individuals captured at each sampling point was determined by direct observation of pleopods and gonopods in females and the presence of genital pores in the coxa of the fifth pereopod of males (Martin & Abele, 1988). Immature individuals with an absence of sexual structures were categorized as recruits of undetermined sex in the field work. To avoid mortality in this endangered species, no individual was transferred to the laboratory or included as a voucher in biological collections. Three body size variables were measured on each individual with a digital caliper of 0.01 mm precision. Caparace length (CL), defined as the distance between the apical end of the rostral spine and the posterior medial edge of the carapace, was considered as the body size variable to control the allometric scaling. The caparace width (CW) was considered as the distance between the left and right points of intersection of the branchial line with the lateral edge of the carapace. The length of the major chela (ChL) was measured as the distance between the anterior end of the closed dactylus and the proximal end of the outer margin of propodus of the first pereiopod. Considering that many of the captured adults presented variable levels of heterochely, whose response can be attributed to regeneration due to autotomy and/or sexual dimorphism (Barría & González, 2008; Trevisan & Santos, 2012), we decided to measure the larger structure to reduce the potential effect of these factors on the observed variation. The sampling and handling procedures of A. concepcionensis individuals were carried out with a research fishing permit granted by the Servicio Nacional de Pesca y Acuicultura de Chile to the first author (Res. Ex.N°1824/2018). The individuals were returned to their habitat when measurement was completed. No individual died due to the stress caused by collection and handling during the entire study period.
The sex ratio of the total sample was calculated monthly and the deviation from a 1:1 ratio was compared with a G-test using Yate's correction (Zar, 1999), which is implemented in the R package DescTools 0.99.36 (Signorell et al., 2020). The adjustment of the frequency of body size to a normal distribution curve was evaluated in different demographic categories (recruits, males, females non-ovigerous and ovigerous) by applying the Shapiro–Wilks normality test (Gill, 1978); the difference in body size between sexes was compared with a non-parametric Mann–Whitney U test (Zar, 1999). Furthermore, to record the number of modal values in the frequency distributions we used the software PeakFit 4.12® SeaSolve Inc., applying residual methods for finding hidden peaks.
The allometric relationships between CW and ChL with respect to CL allowed us to infer body sizes at the beginning of sexual maturity. This assumes that sexual maturity generates changes in the relative growth pattern of body structures which are reflected in the ontogenetic variations experienced by the slope phase line of logarithmic regression models (Somerton, 1980). In females and males separately, the scores of the principal components of the logarithm of the structural measurements (log CW and log ChL) were subjected to Ward's agglomeration algorithm based on Euclidean distances (Murtagh & Legendre, 2014). This is to establish non-hierarchical assignments of individuals to predetermined groups of juveniles and adults. Subsequent discriminant analyses generated functions that allowed classifying any individual of A. concepcionensis into juvenile or adult based on the CW and ChL measurements (Spicer, 2005). The structural growth models of juveniles and adults were compared with a unitary model involving both groups by piecewise linear regression (Vieth, 1989). The significant differences between groups allowed us to adjust the regression equations of both ontogenetic stages and determine a breakpoint in the change of phase line of the slopes (Corgos & Freire, 2006). Ontogenetic and sexual variations in the regression models were compared with two-way analysis of covariance (Quinn & Keough, 2002). We estimated the size at 50% sexual maturity. This parameter corresponds to the median of a cumulative probability distribution of body size of mature individuals and reflects the size at which 50% of a cohort is expected to be mature (Lappalainen et al., 2016). Those individuals belonging to the range of body size where juveniles and adults are mixed were iteratively reassigned to one or another ontogenetic group. In each iteration, the proportion (or probability) of individuals by size interval was adjusted to a logistic function and the value of estimated body size for 50% (or probability 0.5) of the individuals considered mature was interpolated. Thus, we determined a distribution of size values of 50% sexual maturity summarized by the mean and 95% confidence interval (Somerton, 1980). The distributions of males and females were compared with a paired Student's t test for independent samples (Zar, 1999). Relative growth analyses and estimates of size of 50% sexual maturity were performed in the R package sizeMat 1.1.2 (Torrejón-Magallanes, 2019). The seasonal variation in each of the environmental variables was compared with a non-parametric Kruskal–Wallis test followed by a post hoc Dunn's test of significant pairs (Zar, 1999), both implemented in the R package dunn.test 1.3.5 (Dinno, 2015). The association patterns between variables were inferred through a correlation matrix based on Pearson's product-moment correlation coefficient (r). The value of each coefficient and its statistical significance were estimated in the R package corrplot 0.84 (Wei & Simko, 2017). The abundance of recruits, juveniles and adults of both sexes were related to environmental variability separately using multiple regression models based on Bray–Curtis distance matrices (MRM). Due to their low temporal representation, ovigerous females were not considered as an independent comparison group, being included together with the remaining mature females. This method allows each environmental predictor variable to be represented in the model by its own distance matrix, which provides an improved correlation of the species–abundance relationship since the effects of important variables are not diluted by the less important variables (Lichstein, 2007). These analyses were performed using the R package ecodist 2.0.7 (Goslee & Urban, 2007). Finally, the period of embryonic incubation and the onset of the morphological maturity of males and females correspond to the most studied population attributes in the reproductive biology of aeglids. For this reason, we integrate the results observed in A. concepcionensis with those described in other species to propose congeneric patterns of reproductive period and sexual maturity.
3 RESULTS
3.1 Environmental variation
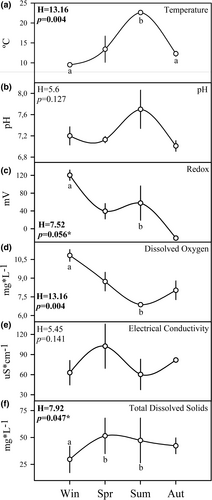
The temporal fluctuation of the environmental variables measured in the water column of the study area presented seasonal patterns of variation. The water temperature presented an average range of 13.1°C during the sampling period, fluctuating between 9.5°C in winter and 22.6°C in summer. The thermal differentials between summer–winter and summer–autumn were the only ones significantly different (Figure 2a). pH had a seasonal pattern similar to that of thermal variation, with a maximum value in summer and an evident decline towards the cold seasons, but without statistically significant differences in the range from pH 7.7 to 7.0 (Figure 2b). The range of the mean redox potential in the water column was 141.4 mV, with a maximum recorded in winter (120.3 mV) and the lowest value in autumn (−21.1 mV). Despite the wide variation observed during the cold seasons, only the difference between winter and summer presented a statistically significant response, this within a hypothesis test that marginally rejected no difference between seasons (Figure 2c). The dissolved oxygen concentration presented an average seasonal range of 3.96 mg*L−1, varying significantly between the maximum value in winter (10.83 mg*L−1) and the minimum value in summer (6.87 mg*L−1; Figure 2d). The seasonality of electrical conductivity showed minimum values in winter and summer and higher values in spring and autumn. The extreme values were recorded in spring (102.5 μs*cm−1) and summer (60.3 μs*cm−1). However, the estimated range (42.2 μs*cm−1) did not present significant differences in the observed seasonal pattern of variation (Figure 2e). The concentration of total suspended solids tended to increase during the warm seasons and to decrease during the cold seasons. The concentration of total dissolved solids had a marginally significant seasonal variation pattern with a mean variation range of 21.8 mg*L−1; the lowest concentration in winter (29.7 mg*L−1) presented more noticeable differences with the highest mean concentrations in spring (51.53 mg*L−1) and summer (47.27 mg*L−1; Figure 2f).
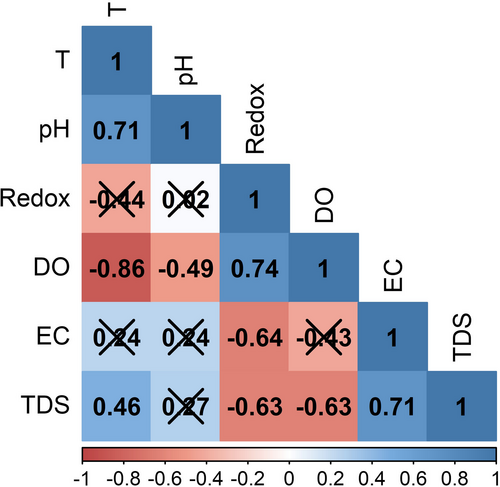
Correlations were low and not statistically significant in six of the 15 paired environmental association patterns (between r = −.44 and r = .27; p > .05; Figure 3). In the remaining comparisons, the paired relationship between temperature-pH, Redox potential-dissolved oxygen and electrical conductivity–total suspended solids presented patterns of direct proportionality with high and statistically significant correlations (r > .7; p < .05). A similar covariation response but with lower correlation was observed in the temperature–total suspended solids association. The temperature-dissolved oxygen relationship was inversely proportional, with a high and significant correlation (r > −.85; p < .05). The covariations between pH-dissolved oxygen, redox potential-electrical conductivity and redox potential–total suspended solids displayed similar response patterns with inverse associations and lower but equally significant correlations (between r = −.49 and r = −.64; p < .05; Figure 3).
3.2 Population structure of Aegla concepcionensis
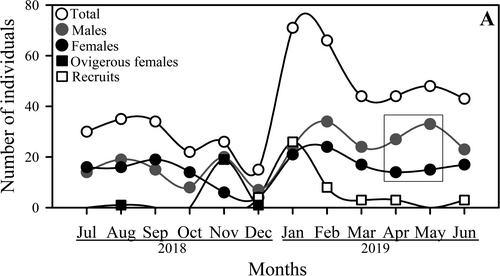
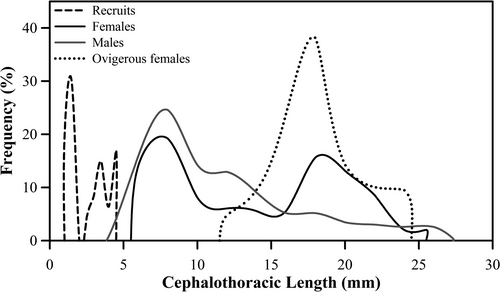
A total of 478 individuals were collected during the study period. The sample included 47 (9.8%) recruits of undetermined sex, 248 (51.9%) males, 162 (33.9%) non-ovigerous females and 21 (4.4%) ovigerous females. The lowest abundance was found on December (15 individuals, ~3.1% of the total), while the highest number of A. concepcionensis (71 individuals, ~16.3% of the total) was recorded in January. The smallest seasonal fluctuation in total abundance was recorded during spring (October–December: 22–15 individuals), followed by winter (July–September: 30–35 individuals) and autumn (April–June: 48–43 individuals), contrasting with the largest fluctuation in total abundance observed during summer (January-March: 71–44 individuals) (Figure 4). The overall male:female ratio was 1.22:1.0; the abundance of females was greater than males only in July, September and October. However, the sex ratio significantly favoured males (G-Test: G0.05,1 = 3.5; p < .05) only in April and May. Ovigerous females were found between August and December, except September and October, with a peak of maximum abundance in November, while recruits of indeterminate sex were observed between December and June, with maximum abundance in January (Figure 4).
The best fitted MRM model based on environmental distance matrices showed that 22% of the A. concepcionensis total abundance was explained by pH as the only variable that contributed significantly to the model (Table 1). pH also contributed significantly to the explanation of the abundance of the juvenile and adult stages of females and the abundance of adults in males, but not with the abundance of juvenile males, whose variation did not present a significantly adjusted multiple regression model. Water temperature contributed significantly to explain the abundance of recruits (Table 1).
| Variable | Females abundance | Males abundance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total abundance | Juvenile | Adult | Juvenile | Adult | Recruits | |||||||
| Coef. | p-value | Coef. | p-value | Coef. | p-value | Coef. | p-value | Coef. | p-value | Coef. | p-value | |
| R2 Model | 0.22 | .05 | 0.2 | .04 | 0.4 | .01 | 0.1 | .33 | 0.3 | .02 | 0.4 | .01 |
| Temperature | 0.16 | .39 | 0.07 | .70 | 0.05 | .75 | 0.11 | .58 | 0.28 | .08 | 0.35 | .032 |
| pH | 0.33 | .05 | 0.35 | .032 | 0.51 | .003 | 0.19 | .25 | 0.27 | .039 | 0.25 | .064 |
| Redox | −0.039 | .72 | 0.022 | .81 | −0.082 | .371 | −0.053 | .68 | 0.24 | .13 | −0.07 | .44 |
| DO | −0.044 | .79 | −0.071 | .65 | −0.002 | .99 | −0.025 | .88 | −0.16 | .12 | −0.08 | .57 |
| EC | −0.230 | .155 | −0.27 | .09 | −0.18 | .19 | −0.20 | .27 | −0.15 | .91 | −0.22 | .083 |
| TDS | 0.183 | .259 | 0.20 | .19 | 0.18 | .19 | 0.17 | .33 | −0.20 | .20 | 0.21 | .12 |
Note
- Bold indicates statistically significant differences (p < .05).
3.3 Body size and relative growth
Cephalothorax length (CL) showed an evident pattern of sexual dimorphism (Figure 5). Males had a CL range of 24.3 mm (between 3.0 and 27.3 mm), which was 12.3% greater than the CL range of 21.1 mm measured in females (between 4.4 and 25.5 mm). The CL frequency distributions differed significantly from a normal distribution (Shapiro–Wilks Test: W = 0.88, p < .001 in both sexes). The PeakFit analysis showed that the distribution of CL values in females had a bimodal structure with two significant peaks of relative frequency at 7.26 mm (19.2%) and at 18.34 mm (15.7%). In contrast, the distribution of CL values in males was unimodal, with maximum relative frequency (24.6%) at 7.8 mm (Figure 5). The dispersion models associated with the mean CL values of females (12.8 ± 5.9 mm) and males (10.6 ± 5.3 mm) presented statistically significant differences [Mann–Whitney U test: Z(U)0.05, (1), 198, 232 = 3.57, p < .05]. The CL values in the recruits fluctuated between 1.0 and 4.4 mm, with a relative frequency distribution that differed significantly from a normal curve (Shapiro–Wilks Test: W = 0.81, p < .0000). Approximately 48% of the recruits presented CL values that fluctuated between 1.0 mm (19.1%) and 1.5 mm (28.9%), while 38.3% of the remaining recruits presented CL values between 3.5 and 4.4 mm. The body size of ovigerous females fluctuated between CL = 12.0 mm and CL = 24 mm. 76.1% of these individuals had CL values between 16.0 and 20.0 mm, with a modal value at 18.0 mm (38.1%; Figure 5). The relative frequency distribution model of CL values of ovigerous females was marginally fitted to a normal curve (Shapiro–Wilks Test: W = 0.92, p = .048).
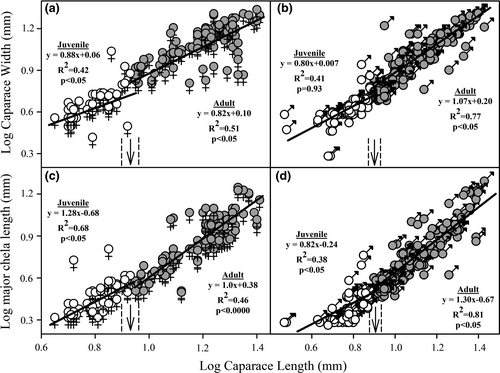
The relative growth models in the length of the major chela (ChL) and caparace width (CW) showed significant variations in the phase line of the linear covariation with the caparace length (CL), which were attributable to ontogenetic differences and not to sexual dimorphism. The slope of the regression models showed significantly different ontogenetic trajectories between juveniles and adults (Figure 6), and the results of the two-way ANCOVA showed that the relative growth was significantly influenced by ontogenetic stage, but not by sex of individuals or by ontogeny*sex interaction (Table 2). More specifically, the growth of CW showed a positive allometric relationship with CL. These associations were significantly adjusted to a linear regression model in females, both juveniles and adults (Figure 6a), and in adult males, but not in juvenile males (Figure 6b). The relative growth of the greater chelipod showed positive and significant allometric patterns with cephalothorax length in both sexes and both ontogenetic stages (Figure 6c,d).
| Source of variation | df | CW | ChL | ||
|---|---|---|---|---|---|
| F | p-value | F | p-value | ||
| Sex | 1 | 0.079 | .78 | 0.078 | .78 |
| Ontogenetic stage | 1 | 11.22 | .0009 | 6.44 | .011 |
| Sex*Ontogeny | 1 | 1.85 | .17 | 0.29 | .58 |
| Error | 438 | ||||
Note
- Bold indicates statistically significant differences (p < .05).
The change of the phase line in the relative growth models was estimated to have occurred at 9.1 mm in females and 8.1 mm in males for both ChL and CW relationship. The trajectories of the growth models showed that in the estimated size of the onset of sexual maturity of both sexes, the expression of CW tended to be more variable due to the potential overlap of juveniles and adults. In contrast, the growth of ChL tended to converge on a value during the onset of sexual maturity (Figure 6c,d). Based on the relationship model between CW and CL, the CL value at which 50% of the females are mature was estimated at 8.5 mm, with a confidence interval between 7.9 and 9.2 mm (Figure 6a,c). On the other hand, the linear relationship between ChL and CL estimated that sexual maturity for 50% of males was at a mean CL value of 7.9 mm and a confidence interval between 7.6 and 8.3 mm (Figure 6b,d). The sexes presented a statistically significant difference in the estimated onset of morphological maturity (t test = −8.17; p < .000).
3.4 Interspecific comparisons
Although we did not find ovigerous females in the September and October samplings, we assumed the possibility of an embryonic incubation period of 5 months in A. concepcionensis, which spanned between mid-winter (August) and the beginning of summer (December). This period was shorter than the mean of 6.2 months estimated for the genus Aegla. Figure 7a shows that the periods of presence of ovigerous females of ≤5 months can be considered infrequent because they were observed in 25% (5 species) of the aeglids studied. The extreme case was even less frequent, since only 15% of the studied species (3 species) had ovigerous females during all months of the year. Therefore, 60% of the studied species had periods of ovigerous females between 6 and 8 months. Thus, A. concepcionensis presented the later period of year of the species with restricted embryonic development, also being one of the few species with the presence of ovigerous females in December. Furthermore, the presence of ovigerous females in the studied species did not show any type of pattern related to latitude (Figure 7a).
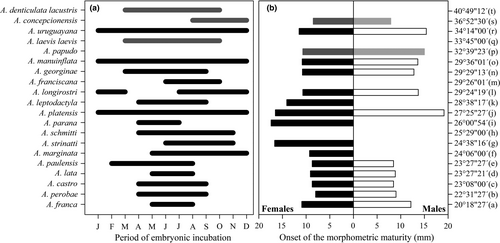
The significantly larger size at the onset of morphological maturity in A. concepcionensis females constitutes an emergent interspecific response. This is due to the fact that size at the beginning of maturity was greater in males in most of the species studied except in A. lata and A. castro, which presented similar sizes in both sexes at morphological maturity (Figure 7b). Males of A. concepcionensis showed the lowest value at the onset of maturity of the entire genus and females the second lowest value after A. perobae; the largest sizes at the beginning of sexual maturity were recorded in species of intermediate latitudes, although the available information provided better signals of this pattern in females (Figure 7b).
4 DISCUSSION
Our results showed that the temporal variations in the population structure and reproductive pattern of A. concepcionensis are attributable to the seasonality of the abiotic environment and the adaptive nature of growth and sexual dimorphism. The seasonal variation and the correlational response of the physicochemical characteristics of the water column in the study area were similar to conditions of good environmental quality of the oligotrophic rivers of Central Chile. Only the mean water temperature in summer (22.6°C) presented a value 2–3°C higher than the nearby records (Correa-Araneda & Salazar, 2014; Debels et al., 2005; Fierro et al., 2012). This thermal difference can be explained because the study site is immersed in an eroded vegetation matrix and severely affected by forest fires (Urrutia-Jalabert et al., 2018), and its riversides are dominated by mainly introduced scarce shrub vegetation that does not generate shadding (Catchpole, S. Unpublished data). The high water temperature apparently does not restrict the ecological performance of A. concepcionensis. In fact, the MRM model indicates that the summer temperature promotes the recruitment of juveniles (Table 2). Our results also suggest that the upper thermal tolerance level (UTT) of A. concepcionensis is greater than 23°C, which is expected for freshwater decapods from temperate zones (Nakata et al., 2002; Stewart et al., 2013). The reduction of the vegetation matrix and direct exposure to solar radiation systematically increase the maximum temperature of the water column of low-order streams (Bunn et al., 1999; Rutherford et al., 2004). This situation generates severe impacts on freshwater benthic macroinvertebrate communities because it selects against those taxa that are more stenothermic and/or that support lower levels of UTT, which include the most endemic and sensitive groups of the orders Ephemeroptera, Plecoptera and Trichoptera, whose UTT levels are ~24°C (Leigh et al., 2015; Stewart et al., 2013), i.e., 1.4°C less than the maximum temperature recorded in this body of water. The MRM model also indicates that the narrow ranges of pH variation measured in the water column can explain the temporary fluctuations of different population components of A. concepcionensis, specifically in the total abundance, the abundance of juvenile and mature females and the abundance of mature males. Our results showed that the water column tended to be slightly more basic in the warmer seasons, which coincided with the periods of greater population abundance. In aquatic environments, small trends towards environmental alkalinity are associated with periods of greater photosynthetic activity and ecosystem productivity (Subramanian & Mahadevan, 1999). And at the organismic level, slight increases in alkalinity strengthen the immunity system of arthropods and optimize some metabolic, physiological and ontogenetic processes (Fadlaoui et al., 2021). On the other hand, the non-significance in the seasonal variation within a range of 0.7 pH units in the study area (Figure 2) is indicative of an environment with a good buffering condition and/or with the absence of important sources of pH variation (Allan & Castillo, 2007; Simonsen & Harremoës, 1978; Welch & Jacoby, 2004). However, this condition can be an unstable equilibrium state because the environmental deterioration of the drainage vegetation matrix can negatively affect the resilience of the buffer effect, leaving the stream exposed to drastic physicochemical changes due to the direct input of terrigenous elements through periodic pulses mediated by precipitation (Pinay et al., 1992; Sabater et al., 2000). Likewise, direct sunlight exposure to watercourses facilitates atmospheric deposition of acidifying substances that reduce pH and promote the loss of freshwater biodiversity (Heino et al., 2009; Pinkney et al., 2015). Thus, deforestation in the drainage area can generate harmful effects on the environment and on the community of this body of freshwater that houses a particularly high abundance of A. concepcionensis.
The presence of ovigerous females during the transition to the warm seasons observed in A. concepcionensis is relevant in the supply of new individuals to the population through the recruitment of juveniles, which was maximized during the month of January, that is, in mid-summer in the temperate zone of the Southern Hemisphere and two months after the maximum peak of ovigerous females observed in November. Therefore, A. concepcionensis would have a comparatively fast embryonic development rate, largely influenced by the trend towards an increase in environmental temperature, which accelerates the metabolic processes associated with organogenesis, embryo growth and yolk consumption (Wehrtmann & López, 2003). This situation is coherent with a gonad cycle model where oocyte maturation occurs during cold seasons, when females increase their per offspring investment due to the optimization of the processes of formation and distribution of yolk in mature oocytes (Thatje & Hall, 2016). Consequently, it is probable that the “reproductive clock” of A. concepcionensis favours the formation of eggs with a structural and energetic quality that promotes an embryonic dynamic whose development rate increases along with ontogenetic advance, resulting in the hatching of juveniles that develop, grow and recruit during the period of greatest environmental productivity.
The greater abundance of Aegla males is a demographic pattern frequently attributed to reproductive behaviours that determine the sex ratio. Some patterns of periodicity in the skew towards males have been explained by the segregation and reclusion of females during the embryonic incubation period and by the deployment of agonistic confrontations between males associated with copulation (Cohen et al., 2011; Swiech-Ayoub & Masunari, 2001; Teodósio & Masunari, 2009). The greater aggressiveness of males has also produced biased sexual proportions as an artefact response when methods based on baited traps or hand nets are used (Masunari, 2019). The application of a sampling effort of A. concepcionensis based on a more representative capture method (discussed below) allowed us to reduce the potential behavioural effect of males in abundance measurements. Thus, it is possible to establish that during the winter-spring period, A. concepcionensis exhibited an unbiased sex ratio with low density and low environmental productivity. However, between summer and autumn the sex ratio tended towards males with significant differences during April–May. This moment is previous to the ovigerous female period and may be related to a greater intensity of agonistic confrontations during the obtaining of mating and copulation pairs (Cohen et al., 2011). Complementary sex-dependent differences were also observed in the size structure of A. concepcionensis. The response patterns support the supply of individuals to the population from recruits of undetermined sex of small body size, which promoted the peak abundance of female and juvenile males of ~8.0 mm cephalothorax length. However, body size structure diverged in both sexes from this threshold. Apparently, females of intermediate size (between 10 and 16 mm CL) were the target of some selection pressure not evaluated in this study, but the peak in abundance of adult females coinciding with the size mode of ovigerous females allows us to infer a selective favouritism of the size fraction with the highest reproductive performance. In contrast, the gradual decrease in the frequency of larger individuals observed in males is consistent with the increase in intraspecific competition and its intensification during the mating period prior to embryo incubation. This implies that the causal mechanisms proposed to explain the demographic response in the size structure are of different selective nature. Since the processes of optimization of oogenesis and reproductive inversion based on the size of the females have direct consequences on population fitness, there may be a strong influence of natural selection on the reproductive success of females. In contrast, the demographic dynamics of males is a response mainly mediated by sexual selection favouring individual fitness through the agonistic encounters during mate acquisition (Kokko et al., 2006; Rice & Chippindale, 2001). The hypothesis of phenotypic sexual dimorphism given by natural selection in females and sexual selection in males has been previously suggested to explain morphological and behavioural differences in aeglids (Barría et al., 2011), and the biological interpretations used to explain the results observed in A. concepcionensis contribute to the generality of this response pattern (Barría et al., 2014).
Modal abundance in both sexes (i.e. juveniles of ~8.0 mm cephalothorax length) coincided with the size range where pubertal moult and the onset of morphometric maturity occur, which is reflected by the significant breaks in relative growth of body structures (Ahl & Laufer, 1996; Hartnoll, 1974). In this sense, A. concepcionensis differs from the rest of the congeneric species by presenting the smallest size at the onset of morphological maturity and a significantly higher value of this variable in females. No morphological divergence was observed in the relative growth of the greater chelipod in A. concepcionensis males. The absence of this dimorphic pattern in males has been described in some species of the same genus, suggesting a growth model with a unique ontogenetic trajectory directed directly towards reproductive functionality (Bueno & Shimizu, 2008, 2009; Chaves et al., 2019; Marçal et al., 2018; Takano et al., 2016).
Compared with congeneric species, the embryonic parental care period of A. concepcionensis was not only restricted and late, but also did not contribute to the support of any of the environmental, climatic or latitudinal hypotheses proposed as predictors of the period of occurrence of ovigerous females (Bueno & Bond-Buckup, 2000; Bueno & Shimizu, 2008; Rocha et al., 2010). We did not find any pattern from the reorganization of the information that coherently relates the embryonic incubation period with the conservation category of the studied species (results not shown). In addition, until now A. concepcionensis is the species where the beginning of sexual maturity occurs in the smallest body size. Thus, the integration of all our results indicates that A. concepcionensis has a population and reproductive activity rate dependent on environmental variability and that tends to increase reproductive development rates instead of body growth, maximizing reproductive efficiency, which is consistent with the proposed prediction. Regardless of whether or not the observed response constitutes an adaptive plasticity strategy in the expression of life-history traits promoted by the selective pressures associated with environmental deterioration (Nussey et al., 2007), the consequent maximization of the resulting reproductive effort may have relevant implications for A. concepcionensis in the context of an adequate local biodiversity conservation programme.
Our sampling method based on low-intensity electroshock was a methodological innovation with respect to the use of traps or hand nets implemented in previous population studies of aeglids (Masunari, 2019). Compared with the more traditional capture techniques, electrofishing improves the representativeness of freshwater macroinvertebrate samples because it promotes the drifting out of all individuals present in the area of influence of the electric shock, reducing the bias by body size or heterogeneity of the habitat generated in the more traditional capture techniques (Lento & Morin, 2014). Furthermore, the sweep of the discharge hoop through the water column also reduces considerably the damage caused by physical disturbances to the microhabitat due to dragging and removal of substrate (Kruzic et al., 2005; Taylor et al., 2001). Despite these methodological advantages, some authors report potentially harmful effects when electrofishing techniques are applied in samples of rare populations and endangered species (Nielsen, 1998; Snyder, 2003). However, the conditions of electrical discharge and sampling effort used in the current study (<30 Hz and <100 watts during 40 min) were previously applied in the capture of live individuals of A. denticulata denticulata (Catchpole & Ruiz, 2018), generating the same floating response of lethargic individuals and subsequent recovery without lethal or sublethal effects. Thus, we suggest that the use of low-intensity electroshocking may be an appropriate sampling method for the in situ population study of aeglid species, especially in those species with critical conservation states.
ACKNOWLEDGEMENTS
We thank two anonymous reviewers for their comments and suggestions, which significantly improved the content of the study. P.S.G. thanks to the National Commission for Scientific and Technological Research for the doctoral scholarship for the financing of his postgraduate studies, CONICYT Doctoral Fellowship 21201107. E.M.B. was funded by CONICYT doctoral fellowship 21161588. R.R. thanks to the Centro de Investigación en Biodiversidad y Ambientes Sustentables, CIBAS, of the Universidad Católica de la Santísima Concepción.




