Long-term trends in the distribution, abundance and impact of native “injurious” weeds
Funding information
The Countryside Survey of 2007 was funded by a partnership of nine government-funded bodies led by the Natural Environment Research Council (NERC) and Defra. Defra funded further work on the analysis of injurious weeds (project WC1042). The research was supported in part by the UK-SCaPE program delivering National Capability (NE/R016429/1) funded by the Natural Environment Research Council. The Biological Records Centre is funded through National Capability funding from the Natural Environment Research Council.
Abstract
Questions
How can we quantify changes in the distribution and abundance of injurious weed species (Senecio jacobaea,Cirsium vulgare,Cirsium arvense,Rumex obtusifolius,Rumex crispus and Urtica dioica), over long time periods at wide geographical scales? What impact do these species have on plant communities? To what extent are changes driven by anthropogenically induced drivers such as disturbance, eutrophication and management?
Location
Great Britain.
Methods
Data from national surveys were used to assess changes in the frequency and abundance of selected weed species between 1978 and 2007. This involved novel method development to create indices of change, and to relate changes in distribution and abundance of these species to plant community diversity and inferred changes in resource availability, disturbance and management.
Results
Three of the six weed species became more widespread in GB over this period and all of them increased in abundance (in grasslands, arable habitats, roadsides and streamsides). Patterns were complex and varied by landscape context and habitat type. For most of the species, there were negative relationships between abundance, total plant species richness, grassland, wetland and woodland indicators. Each individual species responds to a different combination of anthropogenic drivers but disturbance, fertility and livestock management significantly influenced most species.
Conclusions
The increase in frequency and abundance of weeds over decades has implications for landscape-scale plant diversity, fodder yield and livestock health. This includes reductions in plant species richness, loss of valuable habitat specialists and homogenisation of vegetation communities. Increasing land-use intensity, excessive nutrient input, overgrazing, sward damage, poaching and bare ground in fields and undermanagement or too frequent cutting on linear features may have led to increases in weeds. These weeds do have conservation value so we are not advocating eradication, rather co-existence, without dominance. Land management policy needs to adapt to benefit biodiversity and agricultural productivity.
1 INTRODUCTION
Human-induced environmental changes such as eutrophication, land use change, land intensification, disturbance or changes in the management regime may be facilitating the spread of species with the appropriate traits to exploit changing conditions (Smart et al., 2005). This could lead to dominance by aggressive competitive species (“thugs”; Marrs et al., 2011). These are competitors, (Grime et al., 1988), able to colonise disturbed habitats rapidly, exploit unused resources (such as light, nutrients and water; Davis, Thompson, & Grime, 2000) and outcompete existing species. If such a species attains dominance this may lead to filtering of post-disturbance colonists, a decline in patch species richness, loss of smaller, stress-tolerant, low-nutrient species and the creation of homogeneous communities (Smart et al., 2006).
A loss of local i.e. patch-scale, plant species richness has been observed over the past 30 years in Great Britain particularly alongside linear features (streamsides, roadsides, hedges) and in small habitat fragments. At the same time, plant species that are favoured by human activity have become more ubiquitous (Norton et al., 2012). Five native plant species pose a threat to plant species diversity and agricultural productivity and have been classified as “injurious weeds” under the Weeds Act 1959 (with an additional amendment for Ragwort in 2003). These are Common Ragwort (Senecio jacobaea), Spear Thistle (Cirsium vulgare), Creeping Thistle (Cirsium arvense), Broad-leaved Dock (Rumex obtusifolius) and Curled Dock (Rumex crispus). Under this act, land owners can be required through an enforcement notice to take all reasonable steps to prevent spread on their land and onto adjoining land; particularly grazing areas or land which is used to produce conserved forage.
Probably the most controversial of these is Ragwort (Senecio jacobaea). It contains pyrrolizidine alkaloids which are toxic for cattle, horses, other livestock and even humans (Caloni and Cortinovis, 2015). Although livestock tend to avoid it when it is growing in a field, it can be inadvertently cut for hay and included in forage (Laybourn et al., 2013). A number of organizations and individuals advocating Ragwort eradication have claimed it has increased across the British countryside; however, there is a lack of quantitative evidence to test this claim. It is a very visible plant that can be found along roadsides and across fields and perceptions could be distorted by its' high visual impact when flowering.
Cirsium arvense and Cirsium vulgare can be nuisance weeds; their presence in crops leads to yield losses, and in pastures, deterrent leaf spines prevent grazing and utilisation by livestock (Tiley, 2010).
Rumex obtusifolius can decrease the herbage yields and fodder quality in pastures and meadows (Zaller, 2004). Both Rumex obtusifolius and Rumex crispus have been found to cause serious digestive problems for stock if eaten in high quantities (Cavers and Harper, 1964; Hejduk and Doležal, 2004; Kristalova et al., 2011).
We have included an additional species in our analysis: Urtica dioica. It is not classified as an “injurious weed” but is often considered a problem species and has stems and leaves densely covered with stinging hairs, which release potential pain-inducing toxins when brushing contact is made with them and can lead to selective avoidance by livestock (Taylor, 2009).
Although these species are native to the UK they are widespread common nuisance weeds in many countries worldwide (Zaller, 2004; Suter et al., 2007; Tiley, 2010); in addition to the harm caused to livestock and loss of agricultural revenue (Roberts and Pullin, 2007) they can be suppressive and have been found to reduce native plant diversity (Zaller, 2004; Taylor, 2009; Tiley, 2010) although evidence of their impact in the wider countryside is lacking. They are difficult species to manage due to abilities such as production of large numbers of persistent seeds, tolerance to cutting or grazing (regrowth abilities), rapid growth and deep roots (Cavers and Harper, 1964; Zaller, 2004; Suter et al., 2007).
To date, quantitative evidence has been lacking regarding changes in the distribution and abundance of these species and although evidence for the causes of change may be available from small-scale experiments there has been no attempt to determine trends and drivers of change across a wider national landscape. In this study we reviewed the literature describing these weed species and their relationships with potential drivers of change in the British countryside (fertility, disturbance, land management and climate) and used this evidence base to predict responses that were then tested (Appendix S1).
This paper uses data from the Countryside Survey (CS), a unique data set that monitors ecological and land-use change over Great Britain from 1978 to 2007. Fine-scaled data such as these are not available in all countries and may not be available in GB in the future. Current policy and funding is directed towards using unstructured data or creating new monitoring, reliant upon volunteers (Norton, 2018). Therefore, we have also analysed presence/absence data based on occupancy across all hectads in Britain (occurrence within a 10 km × 10 km grid square) collected by volunteers and submitted to the Botanical Society of Britain and Ireland (BSBI), these are the only other national data available over a similar time period.
This study aims: (a) to use existing national data sets to quantitatively assess changes in the frequency and cover of selected native species (injurious weeds) over 30 years in Great Britain; (b) to assess the impact of these changes on plant communities; and (c) to explore the potential to attribute changes in distribution and abundance to explanatory variables and use these relationships to suggest management interventions.
2 METHODS
The study species include: Senecio jacobaea, an herbaceous, winter annual or short-lived perennial that reproduces by seed and vegetatively (Bain, 1991); Cirsium arvense, a perennial herb that can reproduce sexually and regenerate vegetatively from roots (Tiley, 2010); Cirsium vulgare, a biennial or short-lived monocarpic herb, only spread by seeds; Rumex crispus, an annual to short-lived perennial herb that (as Rumex obtusifolius) produces large numbers of seed which remain viable for many years and can regenerate from root fragments (Cavers and Harper, 1964; Grime, et al., 1988); Rumex obtusifolius, less ruderal than Rumex crispus, more robust and exclusively perennial; and Urtica dioica, a tall, usually dioecious, rhizomatous, perennial herb (Taylor, 2009).
The data used in analyses are from the CS of Great Britain and methods are described in detail in Smart et al. (2003) and Norton et al. (2012). The CSs were carried out by professional surveyors in 1978, 1990, 1998 and 2007, and we have used the time series from 1978 to 2007 (and pairs of years inbetween) for this study. The sample design is a series of stratified, randomly selected 1-km2 squares; the number of squares surveyed in 2007 was 591 and the number of squares repeated from 1978 to 2007 was 256. Stratification was based on a classification of all 1-km2 squares using their topographic, climatic and geological attributes. Linear features (road verges, water courses and hedges) were sampled by linear plots (1 m × 10 m) and areal features (fields, unenclosed land) were sampled by five randomly placed plots (200 m2) within each 1 km × 1 km square (Smart et al., 2003). The plots were re-located using metal detectors (to find buried metal plates), photographs and maps.
In each vegetation plot all vascular plants and the more easily identifiable bryophytes were recorded. Nomenclature follows Stace (2010). Cover estimates were made to the nearest 5% for all species reaching at least an estimated 5% cover and marked as present if <5% cover. Each vegetation plot was assigned to a Broad or Priority habitat type at the time of recording (Jackson, 2000).
Presence/absence data based on hectad occupancy (occurrence within a 10 km × 10 km grid square) collected by volunteers (amateur and professional botanists) and submitted to the BSBI were used to compare the use of fine-scaled quadrat data with unstructured data, in part because the fine-scaled data may not be available in the future.
2.1 Changes in frequency (plot occupancy) using CS plot data
Results are presented at national scales for Great Britain (CS and BSBI) and England (CS only).
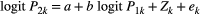
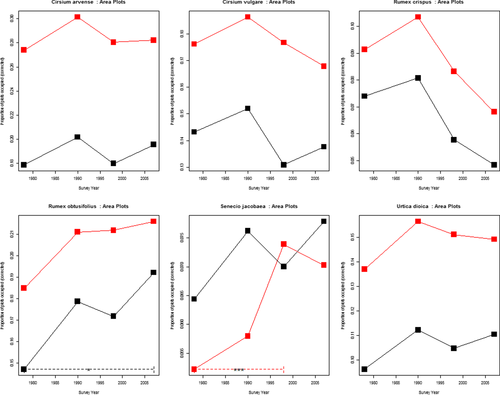
Statistically significant change was attributed to species whose change indices were outside the two-tailed 95 percentile values of all change indices for the subset of data being analysed. Using the adjusted change index estimated with the techniques described above we can achieve corrected occupancy metrics for each weed species which can be seen on the y-axes in Figures 1 and 2 (and Appendices S2 and S3).
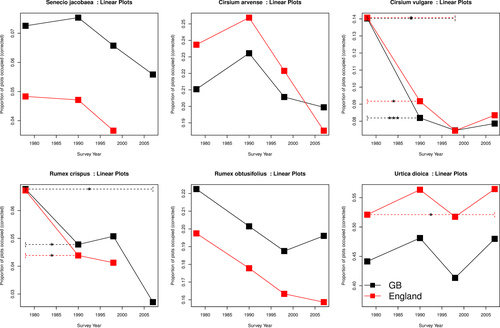
2.2 Changes in frequency (plot occupancy) using volunteer-collected data
Trends in the frequency of the weed species in 10 km × 10 km grid squares across Great Britain were calculated using the Frescalo method (Hill, 2012). This method uses the frequency of locally common benchmark species to adjust for recorder effort, and has been used in several recent analyses of biological records (Pescott et al., 2015); it has also been shown to be generally powerful and robust (Isaac et al., 2014). This method was applied to plant records collected by the BSBI, divided into 5 year periods between 1987 and 2012.
2.3 Changes in abundance (cover) (CS)
The change in cover for each species was estimated by fitting a GLMM in SAS (Enterprise guide version 5.1, © 2012 SAS Institute Inc.) (using proc glimmix-Little et al., 2000) with the 1 km × 1 km CS square incorporated as a random effect to account for non-independence of plots located within the same square. There is a skew in data toward low cover values with relatively few higher cover values so plant cover was square-root-transformed prior to analysis (Ahrens et al., 1990) and the GLMM used a gamma error distribution. Year was coded as a numeric variable to test for an overall significant rate of change across all years rather than testing for separate significant changes between all pairs of years.
2.4 Impact of weeds on vegetation communities
Total plant species richness and the number of ancient woodland, wetland and grassland indicator species were calculated for each plot. Grassland and wetland indicators were identified from Common Standards Monitoring guidance (JNCC-Joint Nature Conservation Committee) and refined in consultation with the Botanical Society of the British Isles to create a list of plants indicative of habitats of high conservation value. Ancient woodland indicators were collated in a separate list from discussions with woodland experts.
Generalised Additive Modelling (GAM; Hastie and Tibishirani, 1990) in R (R Core Team, R Foundation for Statistical Computing, Vienna, AT.) was used (with a Poisson distribution) to analyse interactions between species richness and cover of the weed species and the 1 km × 1 km CS square incorporated as a random effect. The GAMs were plotted to assess the shape and direction of the curve. Upper and lower confidence intervals around the mean trend have been included in Appendix S4. Analyses were carried out using change in weed cover between 1978 and 2007 (Area n = 869, Linear n = 705) and weed cover in 2007 (area plots n = 3,071, linear n = 7,127) as explanatory variables.
2.5 Attribution of species distribution and abundance to explanatory variables
A review of the literature was undertaken to create a series of testable hypotheses (Appendix S1) to explore attribution of frequency and changes in abundance (% cover) to potential drivers of change. The literature suggested that most of the species would be positively or unimodally related to fertility, positively associated with disturbance and grazing (except for Urtica dioica) and that there would be mixed relationships with climatic variables (see Appendix S1 for more detail). Explanatory variables linked to each driver were assembled (Appendix S5). Some of these are proxy variables due to the difficulties in obtaining fine-scaled land management data. Different metrics were used as explanatory variables under the categories disturbance, nutrients, management and climate. Some of these were trait-based measures e.g. Ellenberg scores and canopy height. These were calculated from the vegetation data within a plot where each quadrat was given a cover-weighted mean value for a trait; values were calculated from the vegetation after removing the weed species to avoid circularity. More information on plant trait methods are available in Smart et al. (2005). Other variables were calculated from habitat data within CS and third-party data sources (Appendix S5) as explained below.
2.5.1 Disturbance
Disturbance can be defined as the disruption of the structure of an ecosystem, community or population and changes in resource availability or the physical environment (Turner, 2010). To indicate disturbance, Ellenberg light scores and canopy height were used. High Ellenberg light scores are associated with disturbance and open habitats and low Ellenberg light scores are associated with succession and reduction in incident light at ground level (Weiher et al., 1999). Disturbance is associated with low canopy height (Smart et al., 2005).
2.5.2 Macro-nutrient availability
The Ellenberg fertility score has been used as an indicator of macronutrient availability; higher values are associated with more fertile conditions (Westoby, 1998). The percentage of intensive land (improved grassland and arable habitats) within a square also indicates land use intensity and productivity.
2.5.3 Management
There are considerable overlaps between management activities, disturbance and fertility; however, as we are using different explanatory variables, management has been analysed and discussed separately. Explanatory variables indicating management included whether the land is under an agri-environment scheme agreement (spatial overlay on 1 km × 1 km squares where polygons are in/out of agri-environment options) and the presence of horses, cattle and sheep based on observations made during the survey in 2007 (Wood et al., 2017).
2.5.4 Climate
Climate was used as an additional co-variable to ensure that any observed changes were not simply the result of variation in climatic conditions (Appendix S6).
The explanatory variables described above were used as predictors of the probability of species occurrence in an analysis of 2007 data and a change analysis (1978–2007) in both linear and areal plots in a mixed-model analysis of variance (GLIMMIX procedure in SAS). Each explanatory variable was tested by entering it last into a sequential model after all other variables (Type 1 tests) to quantify the partial explanatory power of each driver and exclude overlapping variance between drivers. A random effect of square was included and, as response data were proportions, a binomial error distribution was used with a logit link function. Model quality verification was carried out using the method proposed by Singer (1998). Akaike Information Criterion (AIC) was used as the model selection criterion; amongst all possible models considered, the one with the smallest value of AIC was considered to be the best model (Ngo and Brand, 2002).
3 RESULTS
3.1 Changes in frequency and abundance
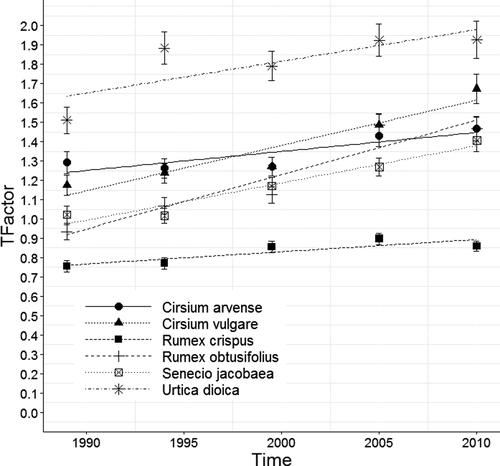
The results from analyses of changes in frequency and abundance can be seen in Table 1 and Figures 1-3. Appendix S3 shows results in more detail with change indices and p values and Appendix S7 shows results for changes in cover in area and linear plots in CS.
|
Trend across area habitats Frequency |
BSBI Frescalo occupancy trends in hectads in GB |
Trend individual habitats or landscape features Frequency |
Trend across area broad habitats Abundance |
Trend individual habitats or landscape features Abundance |
Number of plots (% of total plots) in 2007 | |
|---|---|---|---|---|---|---|
| Cirsium arvense | ns | ↑ 1978 - 2007 GB** and ENG* | ↑Arable**, improved* and neutral grassland* 1978, 1990–2007 GB arable**, acid grassland* 1990–2007 ENG | 2,701 (16%) | ||
| Cirsium vulgare | ↑ 1990–2010 GB** |
↓ Linears 1978–1990, 1998, 2007 GB*, 1978–1990 ENG* ↑ Arable 1990–2007 ENG* |
↑ Streamsides 1990–2007 GB*** and ENG* ↑ Acid grassland 1978, 1990–2007 GB* | 1,219 (7%) | ||
| Rumex crispus | ns |
↓ Linears 1978–2007 GB and 1978–1990 ENG* |
↑1990– 2007 GB and ENG* |
↑ Streamsides 1990–2007 GB* ↑ Neutral grassland 1978, 1990–2007 GB* |
395 (2%) | |
| Rumex obtusifolius |
↑ 1990– 1998 ENG* ↑ 1978– 2007 GB* |
↑ 1990–2010 GB* |
↑ Neutral grasslands 1990 and 2007 ENG** ↑ Improved grassland 1978 and 2007 GB, ENG** |
↑ 1978 - 2007 and 1990–2007 GB** and ENG** |
↑ Linears (streamsides, roadsides, boundaries) 1978–2007 GB*** and ENG* ↑ Improved grassland 1990–2007, 1978–2007, GB** and ENG**, arable 1990–2007 GB** |
2,209 (13%) |
| Senecio jacobaea |
↑ 1978 - 1998 ENG*** ↑ 1990 –1998 ENG*** ↓ 1998–2007 ENG*** |
↑ 1995–2010 GB*** | ↑ 1978 – 2007 GB** | ↑ Roadsides 1990–2007 ENG** and GB** | 737 (4%) | |
| Urtica dioica | ns |
↑ Improved grass 1978–2007 ENG*, 1990–2007 ENG*** and GB** ↑ Acid grass 1978–2007 ENG* ↑ Fen, marsh, swamp 1990–2007 ENG* ↑ Woodland 1978–2007 GB* ↑ Neutral grassland and Urban 1990–2007 GB* ↑ Linears 1978–2007 ENG* |
↑ 1978 – 2007 GB**, 1990–2007 GB** and ENG*** |
↑ Linears 1978–2007***, 1990–2007 GB*** and ENG*** ↑ Improved, acid and neutral grasslands, Fen, marsh, swamp, 1978–2007, 1990–2007 in GB and ENG, woodland 1978–2007 in GB |
4,586 (27%) |
Notes
- Frequency changes are modelled using weed species' counts from the different CS survey years, accounting for recorder effort, and using the residuals from this model as an index of change. Change in cover was estimated by fitting a Generalised Linear Mixed Model (GLMM), year was coded as a numeric variable to test for a significant rate of change across all years. BSBI, Botanical Society of Britain and Ireland.
- * p < 0.05;
- ** p < 0.01;
- *** p < 0.001.
Cirsium arvense did not increase significantly in frequency in the CS or BSBI data. It increased in abundance in the CS data in improved and neutral grassland in GB, acid grassland and arable fields in England between 1978, 1990 and 2007 (Table 1). There were no significant changes along linear features.
Cirsium vulgare increased in arable habitats in England (frequency 1990–2007) and in acid grasslands from 1978 to 2007 (% cover) in GB. BSBI data showed an increase in occupancy (Table 1, Figure 3). Cirsium vulgare declined in frequency along roadsides and hedges from 1978 to 2007 in GB and 1978–1990 in England but increased in abundance on streamsides.
Rumex crispus did not change in frequency significantly in area plots (fields) in CS or in BSBI data. Abundance increased significantly between 1990 and 2007 for GB and England in CS data, particularly in neutral grassland (Table 1). There was a significant decrease in frequency of Rumex crispus on linears 1978–2007 in GB and 1978–1990 in England; however, there were increases in cover on streamsides (1990–2007) in GB (Table 1, Appendix S7).
Rumex obtusifolius has increased significantly in frequency and abundance across England and Great Britain in the past 30 years. This trend was also reflected in hectad occupancy from the BSBI (Table 1, Figure 3). This was particularly in neutral grassland (1990–2007 in England), improved grasslands (1978–2007 in GB and England), arable habitats (1990–2007 GB) and along roadsides, hedgerows, and streamsides 1978–2007 England and GB.
Senecio jacobaea increased significantly in frequency in fields from 1978 to a peak in 1998 in England, since then (1998–2007) it declined (Appendix S2). BSBI data show an increase in GB (Table 1, Figure 3). Abundance increased significantly between 1978 and 2007 in fields in GB and on road verges in England and GB.
Urtica dioica increased significantly in abundance and frequency in fields (1978–2007 in GB and 1990–2007 in GB and England) in many habitat types; Improved, acid, neutral grasslands, fen-marsh-swamp and woodland and in abundance along hedgerows, streamsides, roadsides between 1978 and 2007 in England and GB. This was not reflected in the BSBI results.
3.2 Impact of weeds on vegetation communities
The results from the analyses of cover of weed species against total species richness and indicators (grassland, wetland and woodland) can be seen in Table 2. Overall, change analyses were less significant than the analyses of 2007 data. For some species, there were wide confidence intervals at higher abundances due to lower sample sizes (Appendix S4), although relationships were still significant. In linear plots, there were negative relationships between weed cover of two of the species (Senecio jacobaea, Urtica dioica) and total plant species richness (Table 2; Appendix S4). For indicator species, negative relationships included Senecio jacobaea and Cirsium arvense with grassland and woodland indicators, Rumex crispus and wetland indicators and Urtica dioica with grassland and wetland indicators. There were unimodal relationships between Cirsium arvense, Cirsium vulgare, Rumex obtusifolius, Rumex crispus and total species richness (Appendix S4) and Cirsium arvense, Rumex obtusifolius, Senecio jacobaea and wetland indicators. Rumex crispus was the only species to be positively related to species richness (total, grassland, woodland indicators).
| Cover | Total species richness | Grassland indicators | Wetland indicators | Woodland indicators | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 1978–2007 | 2007 | 1978–2007 | 2007 | 1978–2007 | 2007 | 1978–2007 | |||||||||
| Linear | Area | Linear | Area | Linear | Area | Linear | Area | Linear | Area | Linear | Area | Linear | Area | Linear | Area | |
| Cirsium arvense | ∩* | +*** | ns | ns | −** | ns | ns | ns | ∩*** | +*** | ns | ns | −*** | ∩*** | ns | ns |
| Cirsium vulgare | ∩*** | ns | ns | ∩*** | ns | ns | ns | ∩** | ns | ns | ns | ns | ns | ns | ns | ns |
| Rumex crispus | ∩*** | ∩*** | +** | +*** | +*** | +*** | ns | +* | −*** | ∩* | ns | ns | ns | ns | +* | ns |
| Rumex obtusifolius | ∩*** | ∩*** | ns | ns | ns | ns | ns | ns | ∩*** | ns | ns | +*** | ns | −** | ns | ns |
| Senecio jacobaea | −*** | ∩*** | ns | ∩* | −*** | −*** | ns | ns | ∩*** | ns | ns | ns | −*** | ∩*** | ns | ns |
| Urtica dioica | −*** | ∩*** | −** | + *** | −*** | ns | ns | ns | −** | −*** | ns | +** | ns | −*** | ns | −*** |
Note
- Generalised Additive Modelling (GAM) (Poisson distribution) was used to analyse interactions between species richness and cover of the weed species. ∩= unimodal. Abbreviation: ns = not significant.
- * p < 0.05;
- ** p < 0.01;
- *** p < 0.001.
In area plots, relationships between weed species and total species richness tended to be unimodal or positive. For indicator species, there were negative relationships between Rumex obtusifolius and woodland indicators, Senecio jacobaea and grassland indicators and Urtica dioica with wetland (also positive in change analyses) and woodland indicators. There were unimodal relationships between Cirsium arvense and Senecio jacobaea and woodland indicators, Cirsium vulgare and grassland indicators, Rumex crispus and wetland indicators. Positive relationships includedCirsium arvense and Rumex obtusifolius with wetland indicators and Rumex crispus and grassland indicators.
3.3 Attribution
Appendix S8 shows the results of the spatial (2007) and change (1978–2007) analyses for each species attributed to explanatory variables. Table 3 shows those variables that were significant after model testing and selection using AIC. Most variables remained significant, the exception was sheep for some species.
| Areas | Linears | |||
|---|---|---|---|---|
| 2007 | 1978–2007 | 2007 | 1978–2007 | |
| Cirsium arvense |
+ Disturbance*** + Fertility*** − Moisture*** + Sheep* |
+ Cows*** + Max temp*** |
+ Disturbance*** + Fertility*** + Cows*** + A/E scheme** |
+ In A/E scheme** + Cows* |
| Cirsium vulgare |
+ Disturbance*** − Moisture*** + Cows* + Fertility * |
+ Horses*** |
+ Disturbance*** − Moisture*** + Sheep*** + Fertility** |
|
| Rumex crispus |
+ Disturbance*** + Fertility** |
+ Horses* + Max temp* + Moisture |
+ Disturbance*** + Fertility*** |
|
| Rumex obtusifolius |
+ Disturbance*** + Fertility*** + Cows* − A/E scheme* |
+ Cows*** + Moisture** + Disturbance* + Max temp* |
+ Disturbance*** + Fertility*** − Max temp*** + Moisture*** + Horses** + Cows* |
|
| Senecio jacobaea |
+ Disturbance*** − Moisture*** − Sheep* − A/E scheme* |
+ Horses** |
+ Disturbance*** − Sheep*** − Moisture*** − Fertility* |
− Max temp* |
| Urtica dioica |
− Disturbance*** + Fertility*** + Moisture** + Sheep** |
+ Fertility*** + Max temp** − Disturbance** + Moisture** |
− Disturbance*** + Fertility*** + Sheep*** + Moisture*** |
+ Fertility*** + Max temp*** + In A/E scheme** − Rain* |
Note
- Each explanatory variable was tested by entering it last into a sequential model after all other variables (Type 1 tests) and then significant variables were included in models (null, individual and combinations of variables), the model with the lowest AIC selected and the model components shown in this table. Disturbance is indicated by positive Ellenberg light scores and negative canopy height (analysed separately), Fertility is indicated by positive fertility scores. A/E scheme = agri-environment scheme, Max temp = maximum temperature. Only significant results have been presented. More detailed results can be seen in Appendix S8.
- * p < 0.05;
- ** p < 0.01;
- *** p < 0.001.
3.3.1 Disturbance
There were significant positive relationships between the occurrence of most of the study species in fields and along linear features (Cirsium arvense, Cirsium vulgare, Rumex crispus, Rumex obtusifolius and Senecio jacobaea) and indicators of disturbance (increasing Ellenberg light scores and decreasing canopy height). Urtica dioica was negatively related to disturbance.
3.3.2 Macronutrient availability
Most species (Cirsium arvense, Cirsium vulgare, Rumex crispus, Rumex obtusifolius and Urtica dioica) were positively related to fertility (Ellenberg scores and percentage improved land) in fields and along linear features. Senecio jacobaea was the exception, being negatively related to fertility along linear features. Only Urtica dioica showed a significant relationship with changes in fertility, which was positive for both fields and linear features.
3.3.3 Management
There were positive relationships between Rumex obtusifolius (along linear features) and changes in Cirsium vulgare, Rumex crispus and Senecio jacobaea (in fields) with the presence of horses. Cirsium arvense and Urtica dioica were positively related to the presence of sheep. Senecio jacobaea was negatively related to sheep. Cirsium arvense, Cirsium vulgare and Rumex obtusifolius were positively related to the presence of cows.
Membership of an agri-environment scheme was negatively associated with Rumex obtusifolius and Senecio jacobaea in fields and positively to Cirsium arvense and Urtica dioica along linear features.
3.3.4 Climate
Relationships with climate were not strong. There were relationships between some of the weeds, soil moisture and maximum July temperature (Appendix S8).
4 DISCUSSION
The weed species chosen for this study are fast-growing generalist weed species of concern to policy makers, NGOs and land managers because of the impacts that they have on agricultural systems, livestock health and species diversity.
4.1 Changes in frequency and abundance
Three of the six weed species studied increased in frequency (i.e. the number of plots occupied), over 30 years, indicating that they were invading new habitats. In plots where the weed species were already present, all of the species increased in abundance. This was in many different habitat types: grasslands, arable habitats, roadsides, streamsides and for Urtica dioica wetlands and woodlands. Distribution in landscape features in the neighbourhood of agriculturally managed grasslands can be a significant influence of high weed abundance in these habitats (Suter et al., 2007) so these changes in frequency and distribution in landscape features are important.
Data from two different types of survey were used (CS and BSBI unstructured data). Results from coarser -scale volunteer recording data (BSBI) supported some of the observed changes in frequency in CS (no significant change for Urtica dioica was detected in the BSBI data). CS uses a sample of replicate plots whereas BSBI data come from continuous recording from volunteers where presence is expressed at the 10 × 10 km square scale. Changes in occupancy among hectads requires very common species to undergo huge changes in population size to register an absence, but expansion or contraction at a range edge can be detected. Where common species are abundant, changes in occupancy and cover are more likely to be detected by fine-scaled plots stratified by habitat and landscape features (e.g. CS).
4.2 Impact of weeds on vegetation communities
Many of the weed species studied were negatively associated with plant species richness, particularly along linear features (streamsides, hedgerows and roadsides) but also within fields. This was not only total plant species richness which could indicate a high number of ruderal, competitive, nitrophilous species rather than species of conservation value, but also indicators of higher quality habitats such as grasslands, woodlands and wetlands. Linear features are potentially important refuges for species that have been eliminated from the wider landscape (Smart et al., 2006) and loss of diversity here could have implications for species conservation, resilience and restoration of the countryside.
Within fields there were also some positive and unimodal relationships between weed cover and species richness of all indicator types. Unimodal relationships where species richness is highest at intermediate cover may be associated with disturbance. Highly disturbed, early-successional communities have decreased competition for space and increased resource availability leading to high diversity initially; over time, as a single species dominates, diversity declines (Davis et al., 2005). Rumex crispus tended to be positively or unimodally related to species richness; this may be because Rumex crispus did not reach more than 15% cover in plots so it is unlikely to be having a suppressive effect. Urtica dioica was negatively related to all types of indicator species richness. It is a strong competitor that reduces growth of other species and could lead to monospecific stands.
Competitive interactions between species may not be driving the changes in vegetation communities: the weed species are likely to be “passengers” of change (MacDougall and Turkington, 2005) responding to changes in land use, land management, nutrient input and disturbance. However, the ability of vegetation communities to act as a refuge for species of high conservation value may still be compromised.
4.3 Attribution
In attributing weed dynamics to potential drivers we used a hypothesis-led approach from literature searches of mainly experimental studies. It is important to subject the outcomes of controlled small-scale experiments to testing in the wider countryside to ascertain whether driver–impact relationships are still detectable and to explore multiple drivers at larger spatial scales across gradients of nutrients and management intensity beyond the control of the observer (Smart et al., 2012). Although we have been able to use available data to act as proxy variables for anthropogenic drivers, we would prefer to have more detailed spatially explicit data on land management and nutrient input. However, this is not possible for this scale of data from this time period. The weeds' own traits were excluded from derivations of explanatory variables for fertility and disturbance; however, community dynamics might still mean that the weed species themselves may be exerting an influence on the associated species, which may affect the trait scores.
4.3.1 Disturbance
Disturbance was predicted to be a positive influence on most of the weed species (Appendix S1) and all of the species except Urtica dioica were positively related to Ellenberg light scores and negatively to canopy height. These are pioneer species that require high light intensity, space and limited competition for colonisation and establishment (Watt, 1987; Zaller, 2004; Suter et al., 2007; Martinkova et al., 2009). Disturbance, sward damage and creation of bare ground provide favourable conditions for expansion (van de Voorde et al., 2012). At the seedling stage they are very susceptible to competition from neighbouring plants (Crawley and Nachapong, 1985). They are relatively tolerant to most kinds of disturbances, due to their regrowth abilities (Edwards et al., 2000; Zaller, 2004), and because they are unpalatable to animals (Cavers and Harper, 1964). Maximisation of plant competition may be a good way to control these species (Dauer et al., 2012). Herbicide application is also used as a control measure; however, inappropriate use could exacerbate weed infestations by creating more bare ground (Laybourn et al., 2013). Urtica dioica is a better competitor than the other injurious weeds. It has an exceptionally high relative growth rate and is shade-tolerant so it does not require disturbance to establish and expand (Taylor, 2009). Urtica dioica increased in frequency along linear features, while other species declined; this could be related to increases in woody species, succession and undermanagement (Norton et al., 2012) possibly linked to agri-environment options such as buffer strips. Where they were already established along roadsides and/or streamsides most of the weed species increased in abundance. Many road verges have been subject to too frequent cutting and disturbance which may favour expansion. Less frequent, later cutting (that allows wildflowers to complete their lifecycle) with removal of clippings has been recommended to reduce growth of some of these weeds and improve biodiversity (Bromley et al., 2019).
4.3.2 Macronutrient availability
Responses to nutrient input were predicted to be unimodal or positive because these species exhibit a positive response to fertility but can be outcompeted by other plants that also respond positively (Tiley, 2010). Results showed positive responses for all species except Senecio jacobaea. In experiments, Senecio jacobaea responded to nitrogen addition where there was also disturbance; in fenced dense swards there was only a response when herbicide was applied and reduced the vigour of competing species (Watt, 1987; Suter et al., 2007). Increasing the application of nitrogen may be used as a control measure to reduce the occurrence of ragwort (Suter et al., 2007).
Cirsium arvense is known to prefer fertile substrates and respond well to nitrogen but the response has been found to be more variable when there is competition (Edwards et al., 2000; Tiley, 2010). High applications of fertiliser N have been found to correspond with high incidences of Rumex species in grassland (Peel and Hopkins, 1980; Niggli et al., 1993) and the herbage yield of Rumex obtusifolius increased substantially when nitrogen was added (Kristalova et al., 2011). Reduction in fertility could decrease the abundance of Rumex obtusifolius. Urtica dioica is frequently described as a nitrophile although it can occur on soils in which the supply of inorganic N is adequate for growth (Taylor, 2009).
4.3.3 Management
Although different explanatory variables have been used for management, there is considerable overlap between management activities, disturbance and fertility. Grazing is a form of disturbance, and increased fertility of fields is associated with livestock. Horses, in particular, are selective grazers and create bare patches by eating favoured species (Gibson,1996, Simpson, 1993), avoid unpalatable species (Klinkhamer and Dejong, 1993), and cause soil and sward damage by trampling (Haggar, 1980), horses were positively related to some of the weeds.
The literature shows that grazing effects on Cirsium arvense and Cirsium vulgare are complex; the intensity and timing of the grazing regime are important. Lenient grazing (longer sward height) is associated with decreased weed abundance but heavy grazing (shorter sward height) favours Cirsium arvense and Cirsium vulgare by removing competitors (Bullock et al., 1994; Pywell et al., 2010). Controlling grazing to avoid sward damage, poaching and bare ground should create unfavourable conditions for these weeds. It would be interesting to see the effects of mob grazing which is becoming more widespread (Teague et al., 2013). This is short duration, high intensity grazing, with longer recovery times which should reduce disturbance long-term, create structural and species diversity and increase competition for weeds as well as maintaining fodder yield in relatively productive pastures. This type of grazing may mean that injurious weeds are still present but not at significant abundances. Where the long-term aim is to reduce fertility and restore species-rich habitats then low-intensity grazing with an appropriate grazer may be more suitable. The negative relationship between Senecio jacobaea and sheep is interesting: sheep will eat Senecio jacobaea and their tolerance to pyrrolizidine alkaloids is twenty times that of cattle (Laybourn et al., 2013). It has been suggested that they could be used for control of ragwort.
There were some relationships with climatic variables, (soil moisture and maximum July temperature). However, in general, looking at the number of significant tests (Appendix S8), climate was less important than disturbance, fertility and management (specifically the type of livestock).
5 CONCLUSIONS
Analysis of long-term national data sets suggests that anthropogenic drivers over recent decades may have led to increases in the frequency and abundance of native “injurious” weed species. This appears to have been to the detriment of vegetation communities as there are negative relationships between weed species abundance, plant species richness and indicator species of high conservation value.
Disturbance, grazing (numbers and type of livestock) and fertility have been shown to be more important influencing factors than climatic variables. Poor land management such as overfertilising, overgrazing, soil compaction and sward damage contribute to increases in these weed species in fields and either undermanagement or too frequent cutting along linear features. Measures to control grazing, avoid sward damage, poaching and bare ground and fertilise appropriately in fields and infrequent cutting in spring and late summer along linears should create unfavourable conditions for most of these weeds. Management to reduce the extent of injurious weeds needs to be adapted and well fitted to the overall management objectives for the site and will vary by species. It should also be noted that these weed species do have conservation benefits, and we are not proposing eradication. At low levels, the species can co-exist, as long as they do not expand and dominate.
There is a challenge for land managers to modify prescriptions to reverse these trends to benefit overall biodiversity and agricultural productivity.
ACKNOWLEDGEMENTS
We would like to thank CEH staff and CS surveyors who helped with collection and analysis of CS data, recorders including members of the BSBI who submitted botanical records and Neil Forbes for comments on the manuscript. We thank Peter Rothery and Andy Scott for their original work deriving the statistical model. We would also particularly like to thank Dr Helen Pontier from Defra.
Open Research
DATA AVAILABILITY STATEMENT
Data and reports are available from the CS website: http://www.countrysidesurvey.org.uk/. Landscape area data 2007: https://doi.org/10.5285/bf189c57-61eb-4339-a7b3-d2e81fdde28d; Vegetation plot data 2007: https://doi.org/10.5285/57f97915-8ff1-473b-8c77-2564cbd747bc; Vegetation plot data 1978: https://doi.org/10.5285/67bbfabb-d981-4ced-b7e7-225205de9c96




