Native recolonization following control of invasive Ruellia simplex in a cypress floodplain forest
Abstract
Question
What factors constrain establishment of native plant species after removal of a dominant invasive plant species? Specifically, we tested whether missing soil seed banks, elevated soil nutrients and invasion by other non-native species explained the lack of recovery of native species.
Location
Bald-cypress-dominated (Taxodium distichum) floodplain forest. Paynes Prairie Preserve State Park, Alachua County, FL, US.
Methods
We assayed the plant composition of the soil seed bank to a depth of 5 cm and analysed soil nutrient levels in a floodplain forest invaded by Ruellia simplex. We measured percentage cover of vegetation 1 yr after herbicide application to control R. simplex.
Results
Many native species (largely perennials) and very few R. simplex emerged from the seed bank assay. Herbicide application reduced cover of R. simplex, but 1 yr post-herbicide vegetation cover lacked native perennials, and instead was largely comprised of novel invaders and early successional (annual) natives. Across locations, levels of some soil nutrients (P, Mg) and pH were higher in R. simplex-invaded plots than in uninvaded plots. Similarly, R. simplex-invaded plots were significantly correlated with soil K and Ca levels at some locations but not others.
Conclusion
It seems most probable that watershed-level factors, including soil nutrients and pH, as well as changes in hydrology are interacting with site-level factors, such as remnant invasive non- Ruellia simplex plant propagules and vegetative propagules of R. simplex to constrain long-term native plant community establishment. Propagule limitation from the soil seed bank is not likely a barrier to native plant recolonization in floodplain forests after removal of invasive R. simplex, as diversity and density of native species emerged from the soil seed bank was high and density of R. simplex in the soil seed bank was extremely low. It does seem likely that soil properties are influencing the plant community. A critical next step will be identification of native species tolerant of altered soil and water conditions if restoration efforts are to succeed. However, further research on all spatial levels is needed to effectively restore degraded floodplain forests, especially at the broader watershed level.
Nomenclature
-
- Wunderlin & Hansen (2011)
-
Introduction
Remnant tracts of riparian forest bordered by urban or agricultural systems are critical to managing the quality of natural systems within the larger riparian forest ecosystem as they maintain critical connectivity to larger tracts of riparian forest ecosystems. Remnant tracts adjacent to urban areas act as a filter to reduce urban-sourced contaminants moving into adjacent larger wilderness and more generally act as a buffer between these areas (Tabacchi et al. 2000; Groffman et al. 2003). Riparian forests, specifically, function as nutrient filtration systems, biological corridors and important sources of biodiversity (Naiman & Decamps 1997; Nilsson & Svedmark 2002). Therefore, management efforts to conserve and restore these riparian forests are warranted. However, invasive plant species often challenge these efforts, especially where abiotic conditions have been altered by human activities, because the altered conditions can make it more difficult to control invasive species and establish desirable native species within degraded riparian forest.
Urban development in the vicinity of riparian forests has altered hydrology (Zipperer 2002; Groffman et al. 2003) flooding regimes (Zipperer 2002) and vegetation dynamics (Tabacchi et al. 1998; Groffman et al. 2003), which explain in part why invasive plant species are associated with human-altered watersheds (Lambert et al. 2014). The altered riparian vegetation and hydrologic processes leave floodplains particularly vulnerable to invasion from an abundant supply of invasive plant propagules (Zipperer 2002; Loewenstein & Loewenstein 2005) and nutrient-loaded storm water run-off that promotes their establishment (Zipperer 2002; Elias et al. 2011). Invasive plant species can further alter vegetation dynamics, soil microbial communities and nutrient cycling within an invaded ecosystem (Ehrenfeld 2003). For example, persistent invasion of one introduced species in floodplain forests of southeastern North America, Ligustrum sinense, contributed to reduced native species richness (Merriam & Feil 2002), reduced native seedling growth and survival (Greene & Blossey 2012), as well as altered C:N dynamics (Mitchell et al. 2011).
High soil nutrient levels may provide a mechanism for increased success of invading non-native species and decreased successful establishment of native species in wetlands (McIntyre & Lavorel 1994). For example, in large areas of the Cladium jamaicense-dominated wetlands of the South Florida Everglades, where P levels have increased with agricultural run-off, high nutrient-tolerant Typha sp. have replaced low nutrient-adapted and formerly dominant sawgrass (Richardson et al. 1999). Invasive species can often use nutrient resources more efficiently than native plant species (Baruch & Goldstein 1999; Fisher et al. 2006). Burke & Grime (1996) reported greatest susceptibility to invasion when a disturbance was combined with eutrophication. Similarly, McIntyre & Lavorel (1994) reported that native species richness decreased with increasing soil and water nutrient levels and soil disturbance, while exotic species richness increased with increased water nutrient levels and soil disturbance. Understanding the underlying mechanisms that sustain invasive plants will help to identify effective approaches to enable restoration and prevent the loss of biodiversity and ecosystem functions.
Stream banks and floodplain forests invaded by R. simplex (Syn. Mexican petunia, R. tweediana, R. brittoniana), an introduced herbaceous perennial, is one system to explore the options for restoration of native plant communities in human-altered watersheds. Surveys of natural areas in Florida indicate that R. simplex has invaded stream banks and floodplain forests throughout the state (Hupp 2007) and is currently found in 29 of 67 Florida counties (http://www.plantatlas.usf.edu, Accessed: 15 Jul 2012). It is typically found under narrow hydrologic conditions; it occurs in dense bands between streams and upland (Hupp 2007; Hupp et al. 2009). Ruellia simplex has been listed as a category 1 invasive species with the Florida Exotic Pest Plant Council (http://www.fleppc.org/list/09list.htm, Accessed: Jul 2012), indicating that it is altering native plant communities by ‘displacing native species, changing community structures or ecological functions, or hybridizing with natives’. (http://www.fleppc.org/list/09list.htm, Accessed: Jul 2012). Research indicates that R. simplex can be controlled in the short term (<6 mo) with a single glyphosate application (Reinhardt Adams et al. 2014). However, natural recolonization of native species after herbicide treatments did not appear to result in a diverse native plant community of floodplain characteristic species in the short term (Wiese et al. 2013). Common barriers to recolonization by native plants such as a lack of propagules (Davies & Waite 1998; Fisher et al. 2009; Kettenring & Reinhardt Adams 2011) and altered soil and water nutrient levels (Huenneke et al. 1990; Milberg et al. 1999) may be influencing these outcomes.
Lack of native seed availability in the soil seed bank can limit the recolonization and the tendency of degraded ecosystems to recover without further assistance (Davies & Waite 1998). Native ecosystems with a transient soil seed bank and invaded by introduced plant species tend to produce less native seed to replenish the seed bank compared with uninvaded systems (Hutchings & Booth 1996; Davies & Waite 1998). Additionally, when introduced species produce seed that remains persistent in the seed bank, the presence of non-native seed in the seed bank is increased (Hutchings & Booth 1996). Taken together, seed bank dynamics can dictate restoration outcomes after the removal of invasive plant species from native ecosystems.
Discerning the influence of native propagule limitation and altered soil nutrient levels will help to identify likely restoration outcomes for invaded floodplains in human-altered landscapes. By evaluating site conditions – primarily soil nutrients and soil seed bank composition – and then observing the outcome of invasive removal, we can provide evidence that supports the relative importance of each of these barriers to preventing restoration of the native plant community. Our objective was to evaluate the restoration potential of a R. simplex-invaded floodplain forest ecosystem by identifying potential barriers to native recolonization after R. simplex control in the R simplex-invaded bands. Specifically, we examined the differences in four factors between the invaded and uninvaded bands: (1) plant species composition of the soil seed bank, (2) soil nutrient levels, and (3) composition of above-ground vegetation cover.
Methods
Study site
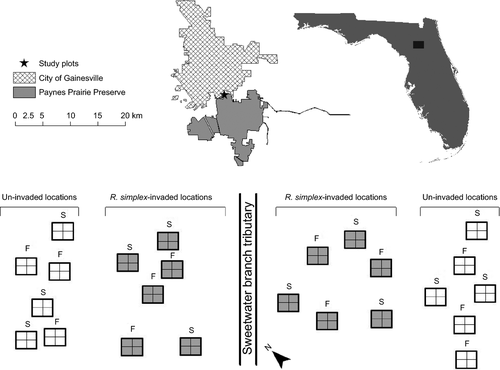
The study site was a bald-cypress-dominated (Taxodium distichum) floodplain forest located in Paynes Prairie Preserve State Park, Alachua County, FL (29°37′21.7″ N, 82°19′20.8″ W; Fig. 1). The soil was predominantly in the mulat sand (loamy, siliceous, subactive, thermic Arenic Endoaquults) series (http://websoilsurvey.nrcs.usda.gov, Accessed: 20 Apr 2012). The study area was bisected by the Sweetwater Branch tributary (referred to hereafter as the stream), which was supplied with storm-water run-off from nearby urban areas. Ruellia simplex typically establishes in different hydrologic conditions than adjacent uninvaded vegetation (Hupp 2007; Hupp et al. 2009). As is typical, R. simplex at our study site was found in broad bands between the stream and more upland areas. Prior to removal of R. simplex, its cover ranged from 75–100% in the invaded bands within the study area (Reinhardt Adams et al. 2014). Adjacent to the bands of R. simplex, native vegetation was predominantly early succession annual and perennial herbaceous species that spread by seed. The majority of native species present produces their seed rain in the autumn, which coincides with the Florida dry season, and produces seed that is persistent in the seed bank (Hupp 2007).
Experimental design and sample collection
Six 3 × 3 m plots were randomly located in R. simplex-invaded bands on both sides of the stream bisecting the study site (i.e. 12 invaded plots in total; Fig. 1). Six additional plots were established in adjacent upland areas where R. simplex had not invaded on each side of the stream bisecting the study site (i.e. 12 uninvaded plots in total; Fig. 1). Uninvaded sampling locations were located 3–12 m away from the edge of the R. simplex-invaded bands (Fig. 1). To facilitate the collection of representative soil seed bank and soil samples, each plot was divided into four 1.5 × 1.5 m subplots.
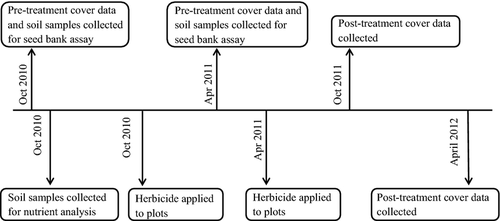
Soil samples were collected from all 12 invaded and 12 uninvaded plots for soil nutrient analyses and seed bank assays. Soil samples were collected in Oct 2010 (autumn samples) for soil nutrient analyses and the seed bank assay, and again in Mar 2011, from different plots (spring samples) for the seed bank assay (Fig. 2). For each soil sample, four subsamples were collected from each subplot, and combined (i.e. one per plot). Soil samples for the seed bank assay were collected with a 5.5-cm soil corer to a depth of 5 cm. All extant vegetation was removed with a foliar application of a broad-spectrum herbicide (a 2% solution of glyphosate, Roundup WeatherMAX®, 48.8% a.i., Monsanto, St. Louis, MO, US, at a rate of 11 206 l·ha−1) to control R. simplex in invaded plots after soil samples were collected.
Seed bank composition
The seedling emergence method was used to evaluate species diversity and density of germinable seeds in the soil seed bank. Seed bank samples were sieved through a 6.35-mm wire mesh and all visible vegetative material was removed. Sturdy plastic trays (Permanest, Growers Supply Company, Dexter, MI, US), 20 × 20 × 6 cm, were drilled with five 0.64-cm drainage holes in the bottom and were lined with a 2-cm layer of coconut coir (Sunleaves Garden Products, Bloomington, IN, US). The coconut coir was covered with a 2-cm layer of Fafard #2 soil mix (Conrad Fafard, Inc., Agawam, MA, US). A 250-ml subsample of each pooled and sieved seed bank sample was spread evenly over the top on the Fafard #2 soil layer. All trays were randomly located on a ponded greenhouse bench under 400 W HID lighting (Model S51; Lithonia Lighting, Conyers, GA, US) on a 14-h day cycle. Four ‘blank’ control trays containing only coconut coir and Fafard #2 soil mix were randomly located on the greenhouse bench between those trays with seed bank samples to assess any possible seed contamination. Water levels were maintained at a constant depth between 0–1 cm of the soil surface using an automatic stock tank siphon float valve (Model M1/4TF mini cooler valve; Watts Water Technologies, North Andover, MA, US). When seedlings were large enough to survive transplant, they were transplanted into azalea pots that were 10.16-cm diameter and 6.99-cm tall (Horticultural Products, Inc., Waco, TX, US; one seedling per pot) containing Fafard #2 soil mix and maintained on a ponded greenhouse bench. Seedlings were identified to species when they had produced reproductive material or were otherwise identifiable. Both the autumn and spring seed bank assays ran for 6 mo. Number of actual seedlings germinated from soil samples was converted to number of seeds per m2 in a 5-cm layer to evaluate total and native seed density. Total species richness, native species richness, total seed density, native seed density and the Floristic Assessment Quotient for Wetlands (FAQWet) indices (Ervin et al. 2006) were calculated for each treatment. FAQWet index is a score that can be calculated and used to evaluate the overall floristic quality of wetland habitats when coefficients of conservatism are unavailable for a site (Ervin et al. 2006). It is calculated as FAQWet = (ΣWC/√S) × (N/S), where WC is the wetness coefficient for native species, N is the number of native species, and S is the total species richness. Wetness coefficient is a numerical representation from −5 to 5 of wetland indicator status as defined by the USDA Plants Database (http://plants.usda.gov).
Vegetation percentage cover
Percentage cover of each plant species was measured in each of 12 Ruellia-invaded plots before and after herbicide treatments (Reinhardt Adams et al. 2014; Fig. 2). Three invaded plots at each location were measured in each season (autumn and spring). Plots were randomly assigned to either autumn or spring using a random number generator program. Percentage cover of vegetation was measured before glyphosate application and at 1 yr after glyphosate treatment (Reinhardt Adams et al. 2014; Fig. 2) Pre- and post-herbicide percentage plant cover was measured in Oct 2010 and 2011 (autumn plots) and Apr 2011 and 2012 (spring plots; Fig. 2). Percentage cover class was recorded using the following modified Mueller-Dombois scale: 0 = 0%, 1 = <1%, 2 = 1–4%, 3 = 5–24%, 4 = 25–49%, 5 = 50–74%, 6 = 75–94% or 7 = 95–100% (Mueller-Dombois & Ellenberg 1974). Plant percentage cover data reflect total plant cover measured over multiple strata.
Soil properties
All soil samples for nutrient analyses were air-dried, ground and passed through a 2-mm sieve. Soil samples were analysed for pH, P, total Kjehldahl N (TKN), nitrate-nitrite, K, Mg, Ca and organic matter content. Soil nutrient analysis was completed by UF-IFAS Analytical Research Laboratories (Gainesville, FL, IS).
Data analysis
The experiment was a completely randomized design, so data from the seed bank assays and soil nutrient analyses were analysed with ANOVA in SAS PROC MIXED (v 9.2; SAS Institute Inc., Cary, NC, US). The three factors used to assess differences in density and diversity in the seed bank experiment were: season (autumn or spring), location (SE or NW side of the stream), and above-ground presence of R. simplex (invaded or uninvaded). The two factors used to assess differences in soil pH, organic matter and nutrients in the soil nutrient experiment were: location (SE or NW side of the stream) and presence of R. simplex in above-ground cover (invaded or uninvaded). The model residuals were checked for normality with histogram and normality plots. Pair-wise comparisons were completed using Tukey's honestly significant difference (HSD) test at a significance level of P < 0.05.
Non-metric multidimensional scaling (NMS) was used to examine patterns in plant species composition of the soil seed bank and above-ground species percentage cover with environmental variables (soil properties) at both plot locations (NW and SE). Density of each species present was plotted in multi-dimensional space. NMS was also used to analyse patterns in seed bank species composition when R. simplex was either present or absent in the above-ground cover. Ordinations were run using PC-ORD 6.0 (MjM Software Design, Gleneden Beach, OR, US). The number of iterations was 500 and the Bray-Curtis dissimilarity measure was used for each matrix.
The Spearman rank correlation was used to evaluate similarity between species composition of seed bank and species composition of above-ground cover. Correlation between seed bank and above-ground cover of native species richness, total species richness and FAQWet index was analysed both before and 1 yr after herbicide treatments, in two seasons (autumn and spring) and at two locations (NW and SE sides of the stream).
Results
Species composition of soil seed bank and above-ground plant cover
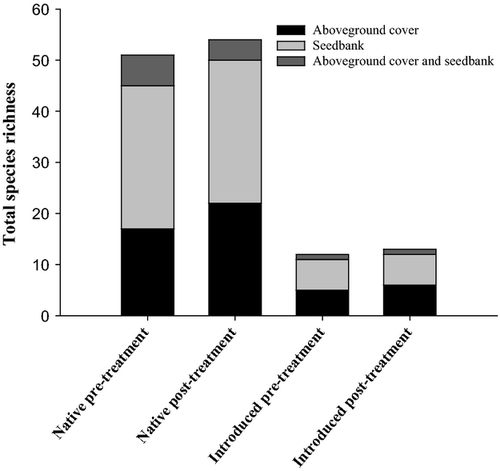
A high number and density of native species emerged from the seed bank, and there was little emergence of R. simplex. Across all plots and treatments, 70 (autumn) and 38 (spring) total species germinated in the seed bank assays under greenhouse conditions, of which 58 (autumn) and 31 (spring) were native. Total seed bank density across all plots and treatments was 83 600 seed·m−2 from autumn and 37 600 seed·m−2 from spring-collected samples, of which 70 400 seed·m−2 (autumn) and 31 800 seed·m−2 (spring) were from native species. More than 50% of species that germinated from the seed bank were native herbaceous perennials, in both seasons, and likely to maintain a persistent soil seed bank (i.e. beyond one annual cycle). The most abundant species which germinated from the seed bank were primarily early succession perennial native species including: Polypremum procumbens, Eupatorium capillifolium and Ludwigia palustris. Introduced species (herbaceous annuals, herbaceous perennials and woody perennials) made up <25% of seedlings in both seasons. Ruellia simplex seedlings in both seasons germinated only from samples collected from a single plot located on the SE side of the stream where R. simplex had been present prior to herbicide application.
Soil seed bank metrics were influenced by sample location and season, but not by presence of R. simplex in the plant cover. Total and native species richness of the seed bank (Fig. 3) as well as total and native seed bank density were higher for samples collected in the autumn from the SE side of the stream than from all other season × location combinations. ‘Quality’ of the seed bank as determined by the FAQWet index (Ervin et al. 2006) was higher in samples collected from the SE side of the stream than from the NW side in both autumn (1.90 vs −0.12) and spring (3.03 vs −0.68).
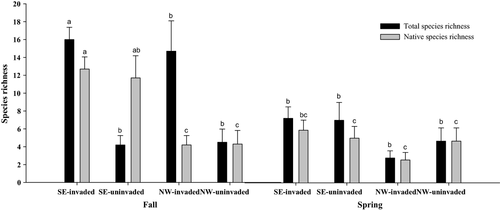
There was little correlation between species composition (native species richness, total species richness) or quality (FAQWet index) of seed bank and above-ground plant cover (Figs 4 and 5). Although R. simplex cover was reduced significantly after herbicide treatments (Reinhardt Adams et al. 2014; this study), above-ground cover at 1 yr after herbicide application was composed to a large degree of novel invaders (primarily Tradescantia fluminensis and Colocasia esculentum) and early successional natives (e.g. Acer negundo seedlings) (Appendices S1 and S2). At the SE location, species composition of seed bank and above-ground cover in the spring at 1 yr post-herbicide treatment were correlated (total species richness, P = 0.0235, r = 0.64499; native species richness, P = 0.0114, r = 0.69918) as was species composition of seed bank and above-ground cover in the autumn before herbicide treatment (total species richness, P = 0.0479, r = 0.58025; native species richness, P = 0.0463, r = 0.58372). Quality of seed bank and above-ground cover was also correlated at the SE location at 1 yr post-herbicide treatment (spring, P = 0.0142, r = 0.68386; autumn, P = 0.0430, r = −0.59102). There were no other correlations between species composition and quality of seed bank and above-ground cover.
Soil properties
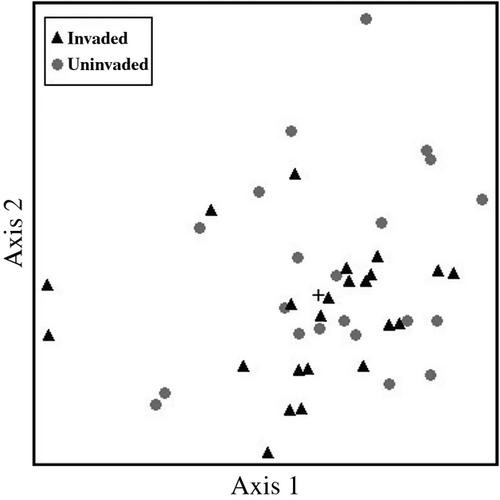
High levels of some soil nutrients were related to the presence of R. simplex (Table 1). Levels of initial P, Mg and pH were higher in plots with initial presence of R. simplex, but they were not affected by location (side of Sweetwater Branch tributary; Table 1). Soil K levels were higher in the R. simplex × NW location treatment or the No R. simplex × SE location treatment than No R. simplex × NW location (Table 1). Soil Ca levels were higher in the Ruellia × NW location treatment than in all other treatments (Table 1). Non-metric multidimensional scaling ordination showed that species recorded from above-ground cover and seed bank were grouped according to location treatment (NW or SE) along a Ca gradient. Levels of total oxidized N (NOxN), percentage organic matter and TKN ranged from 2.79–5.57 mg·kg−1, 7.36–11.36% and 2308.67–3833.0 mg·kg−1, respectively, and were not different between treatments.
| R. simplex Invaded | No | Yes | ||||||
|---|---|---|---|---|---|---|---|---|
| Location | NWa | ±SE | SE | ±SE | NW | ±SE | SE | ±SE |
| pH* | 7.0 b | 0.18 | 7.20 b | 0.14 | 7.70 a | 0.04 | 7.70 a | 0.06 |
| P* (mg·kg−1) | 111.76 b | 17.38 | 159.37 b | 13.69 | 203.20 a | 16.53 | 198.42 a | 7.32 |
| K (mg·kg−1) | 65.86 b | 7.35 | 130.97 a | 22.65 | 134.27 a | 14.61 | 107.65 ab | 13.04 |
| Ca (mg·kg−1) | 2970 b | 594.86 | 4947 b | 586.26 | 13977 a | 2349.93 | 5890 b | 224.84 |
| Mg* (mg·kg−1) | 355.37 b | 46.63 | 448.77 b | 118.04 | 685.28 a | 61.15 | 528.40 a | 26.43 |
- a Letters denote differences between treatments at P < 0.05. Treatment means with the same letters are not significantly different.
Discussion
Based on past research, we did not expect that native recolonization would occur; as invasive species control alone was not previously sufficient to restore R. simplex-invaded floodplain forests elsewhere (Hupp 2007; see Wiese et al. 2013). While experiments show that control of R. simplex can be achieved in the short term with herbicide treatment (Reinhardt Adams et al. 2014; this study), subsequent native recolonization was not adequate to produce a diverse community of plant species, at least within the first year after invader control. In other systems, both elevated soil nutrients and depauperate soil seed banks have precluded transition to native species dominance following invader control (Gerritsen & Greening 1989; Bakker & Berendse 1999). Ruellia simplex-invaded and uninvaded bands are generally found in differing hydrologic zones (Hupp 2007; Hupp et al. 2009), therefore a number of abiotic and biotic factors are likely influencing the presence or absence of invasion, including water availability, soil nutrient properties and native plant propagule availability.
Species composition of the seed bank suggests that this is not a barrier to native recolonization of R. simplex-invaded floodplain forest. A high number of native species emerged from the seed bank, of which more than 50% were native herbaceous perennials in both seasons of seed bank collection. The presence of a high percentage of native perennials may increase the potential for restoring the native community from the soil seed bank since perennials can often better withstand early season flooding or more frequent flooding than native annuals (Abernathy & Willby 1999). Introduced species (herbaceous annuals, herbaceous perennials and woody perennials) made up less than 25% of seedlings in both seasons. These findings are in contrast to Holmes (2002) who reported that when above-ground vegetation in a South African fynbos habitat was primarily invasive species, the guild structure of the seed bank was dominated by short-lived and/or invasive species, specifically annuals, geophytes and short-lived shrubs.
While a large number of species that emerged from the seed bank were native, the seasonal differences in total and native species richness suggest that total and native species richness in the autumn compared with spring may not be an accurate indication of species available in the seed bank for competition with R. simplex. While R. simplex is perennial, it typically dies back significantly in the winter season (C. Reinhardt Adams, pers obs). Native species that establish during the early spring window before the seasonal resurgence of R. simplex growth would likely have the best opportunity for outcompeting R. simplex. We suspect that the higher total and native species richness of the seed bank recorded in the autumn may be a measure of seed rain plus those species persistent in the seed bank. However, some of the seasonal differences in the soil seed bank may be due to the spatial variation between plots sampled in the autumn vs those different plots sampled in the spring. Total and native species richness recorded from spring-collected samples may be a better measure of species persistent in the seed bank, i.e. remaining in the seed bank after 1 yr (Thompson & Grime 1979). However, while some of these species do not seem to be persistent in the seed bank, species that are prolific in the seed rain could also contribute to recovery of the native plant community. Schneider & Sharitz (1986) similarly noted that seed density (both woody and herbaceous species) in the seed bank changed seasonally; soil seed bank density increased after autumn seed rain and decreased when spring rains began. Likewise, Thompson & Grime (1979) reported seasonal differences in species density in the soil seed bank; in particular soil seed bank density of perennial grasses had a clear peak in late spring and summer.
The low germination of R. simplex from the seed bank suggests that its presence in the seed bank is not providing a barrier to restoration. Ruellia simplex germination from seed bank samples was surprisingly low, given that preliminary studies have suggested that R. simplex's abundant seed production, lack of primary dormancy and seed longevity were a potential source of rapid R. simplex invasion (Wilson & Mecca 2003). Ruellia simplex seed reportedly has high viability upon initial dehiscence from the parent plant, and high germination rates regardless of soil type or flooding conditions when viable (Wilson & Mecca 2003). While it is possible low germination of R. simplex in the greenhouse was due to not replicating field conditions sufficiently to support germination, viability in the field was reduced fairly rapidly after the first 13–15 mo (Hupp 2007), which may explain the low R. simplex germination rates we observed in the greenhouse.
As expected, we saw significant differences in species composition of the seed bank compared to species composition of the above-ground plant cover. Seed banks typically have differences in species composition when compared to above-ground vegetation (Thompson & Grime 1979; Davies & Waite 1998; Abernathy & Willby 1999; Bakker & Berendse 1999). Changes in seed rain and seed survival over time can contribute to differences in species composition of seed bank compared to that of above-ground cover (Thompson 1986). Additionally, both rare species and species very common in the above-ground vegetation often exist at a lower percentage of total species composition in the seed bank; any species with transient soil seed banks are also often under-represented in seed bank assays (Bakker & Berendse 1999).
The high soil nutrient levels may have selected for a certain palate of tolerant species, such as the primarily early succession native annuals and perennials as well as novel invaders (Appendices S1 and S2) that were present. Efficiency of nutrient use, a trait linked to invasibility of some species, and high soil nutrient levels may explain why so many of the species represented in the higher species richness of the seed bank were not present in the vegetation cover (King & Buckney 2000; Koutroubas et al. 2000). Anaerobic soil conditions, like those found in floodplain forest soils, can absorb more P from high-P solutions (Patrick & Khalid 1974), like soil water that has increased levels resulting from urban run-off. Levels of Ca, Mg and pH can further influence soil P exchange, resulting in altered soil nutrient conditions (Patrick & Khalid 1974). King & Buckney (2000) similarly found that although native species were present at sites supplied with urban stormwater run-off, an altered plant community was present, likely because the higher nutrient levels selected for the native species tolerant of these altered conditions. Koutroubas et al. (2000) reported similar findings from their study of several herbaceous perennial species in a greenhouse study; species that could more efficiently use a limiting nutrient had a competitive advantage over those species with lower nutrient use efficiency within a low-nutrient habitat or plant community. However, changing water levels are part of the normal hydrology of stream communities (King & Buckney 2000), so some changes in native plant composition are to be expected and would not necessarily be attributable to changes in nutrient levels. While it can be difficult to separate the direct impact of soil nutrients from hydrological changes on invasive species, separating the two is not necessarily critical, as land managers must work with their combined effects.
Ruellia simplex invasion of the floodplain forest site may also be related specifically to interactions between soil pH, P, Ca and Mg supplied by stormwater run-off from urban areas. Soil nutrient levels (P, Mg, K and Ca) and pH were higher in areas with R. simplex cover (50–100%) compared with adjacent areas with no R. simplex cover. Similarly to our findings, others found differences in soil nutrient properties between R. simplex-invaded and uninvaded areas; for example, higher pH and readily exchangeable Ca were reported in R. simplex-invaded areas compared to uninvaded adjacent upland and submerged zones (Hupp 2007). Likewise, higher use efficiency of N and P by shoots and K by roots was observed when R. simplex was grown under wet conditions compared to dry conditions (Wilson et al. 2004). Dense stands of R. simplex may indicate not just that the species is more tolerant of high nutrient levels and pH than other species, but that it is able to thrive under these conditions and therefore able to out-complete other species, both native and exotic. These broad patterns suggest soil properties should be investigated further as a driver of invasion, particularly since ordination suggests R. simplex can invade regardless of species waiting to recruit from the soil seed bank.
It is also possible that the presence of R. simplex is in some way producing a localized increase in some soil nutrients and pH. Fox & Kitajima (2004) found a higher N content in R. simplex tissue compared to co-occurring native species, and suggest that where R. simplex had invaded, it may have been having a localized impact on nutrient cycling. Others have found a similar scenario with other species, for example, a localized increase in N beneath invasive Lantana camara under field conditions and high pH under invasive Berberis thunbergii and Microstegium viminium compared to soil under nearby native Vaccinium pallidum (Ehrenfeld et al. 2001). These differences between the soil nutrient properties of soil under invasive vs native species have been replicated under controlled greenhouse conditions in several cases (Ehrenfeld et al. 2001; McGrath & Binkley 2009). Further research will need to be done to evaluate to what extent R. simplex may be locally altering soil chemistry and creating conditions that support its aggressive growth.
Conclusions
High native species richness among seedlings germinated from the seed bank suggests lack of native propagule availability is not a barrier to native plant recolonization in cypress-dominated floodplain forest. Although the lower native species richness in the spring is likely the more accurate representation of species that remain persistent in the seed bank, compared to the species richness recorded in the autumn, which includes both seasonal seed rain and species that persist in the soil seed bank, native species richness was still relatively high, suggesting the available species pool was not preventing native plant recolonization. Elevated soil and water nutrient levels may, however, provide a barrier to recolonization of native vegetation. It is unclear whether the presence and interaction of high soil nutrient levels, in particular, Ca, are contributing to the establishment of R. simplex and dense cover of other invasive species or whether these conditions, possibly in combination with other factors, are inhibiting germination of native seed present in the seed bank. Additionally, it is unknown whether high pH under R. simplex cover is influenced by the presence of R. simplex or whether R. simplex is more tolerant than native species of high pH soils, possibly as a result of accumulation of high pH sediment from storm water run-off.
Native recolonization of a cypress-dominated floodplain forest, after invasive species control, is likely to be dependent upon both native propagules available in the seed bank and their tolerance to altered soil and water nutrient levels. Therefore, native recolonization will likely require that ecosystem conditions at the watershed level (e.g. soil and water nutrient levels) as well as at the site level (e.g. on-site remnants of exotic plant populations and propagules) be addressed. The contribution of seed rain to restoration outcomes after removal of the invasive species also requires more research. Identifying nutrient inputs from upstream sources will likely be a critical part of long-term restoration of R. simplex-invaded sites. Additionally, other abiotic and biotic factors may differ between the invaded bands and uninvaded bands, which could influence the on-going persistence of a newly restored native plant community. Additional experimental research under controlled conditions (e.g. greenhouse or mesocosm) is needed to identify native species that can establish in the soil conditions associated with newly cleared areas of R. simplex now that some of these conditions have been identified.
Acknowledgements
The USDA T-STAR Program provided financial support for this research. We would like to thank Alice Rankeillor (Gainesville Regional Utilities) for providing field site access for this research. We thank Dr. Jared Westbrook for assistance with statistical analysis and for a helpful review of an earlier draft of this manuscript. We also thank Dr. Andrew Koeser for review of an earlier draft of the manuscript, William Mazzota for assistance in data collection, and Dr. Lucas Majure for expertise in plant identification.