Defining a safe corridor for trans-iliac pin placement in cats
Abstract
Objective
To identify whether a theoretical predictable safe corridor is available in cats for placement of trans-iliac pins without the use of fluoroscopy.
Methods
Twenty-one cats with straight orthogonal normal pelvic radiographs were included. Two start points were evaluated: a midpoint and a dorsal point. The midpoint was defined as midway between the dorsal lamina of the sacral vertebral canal and the cranial dorsal iliac spine. The dorsal start point was 2 mm ventral to the cranial dorsal iliac spine. The pin was assumed to be driven at 90 degrees to the lateral face of the ilium, and considered surgeon accuracy was ±4 degrees from the perpendicular. The angular range and the distance between the iliac wings from the ventrodorsal radiograph were used to calculate the possible cross-sectional area and pin exit location if driven from one iliac wing to the other. The corridor was then evaluated for repeatability in six randomly selected cats.
Results
Vertebral foramina penetration risk was identified in some cats when using a 1.6 and 2 mm-diameter pin using the mid-iliac wing start point. The dorsal start point decreased the available pin placement area but reduced the risk of entering the hazardous zone for all pin sizes up to 2 mm.
Conclusion and Relevance
A theoretical defined safe corridor is available for trans-iliac pin placement in cats between 2.0 and 5.5 kg. A 1.2-mm pin is the safest if using the mid-iliac wing start point. A more dorsal start point can accommodate up to a 2.0-mm pin if correctly aligned to the sacrum.
Abbreviations
-
- dHPC
-
- hypothetical placement corridor
-
- DLVC-I
-
- dorsal lamina of vertebral canal to cranial dorsal iliac spine
-
- HPA
-
- hypothetical cross-sectional area
-
- IWA
-
- iliac wing area
-
- ML
-
- mediolateral
-
- SI
-
- sacroiliac
-
- VD
-
- ventrodorsal
Pelvic fractures are common, accounting for 20%–32% of cat fractures, and 60% of these have a sacroiliac (SI) fracture luxation.1-3 Unilateral SI luxation most commonly presents with other pelvic fractures (pelvic floor fracture and contralateral iliac body fracture) or symphyseal separation,3 whereas bilateral SI luxation may occur in isolation.2
Treatment options for SI luxation range from conservative management to bilateral surgical intervention. Conservative treatment includes pain management, cage rest and monitoring the ability to urinate and defecate appropriately.4 It is often used in less severely affected patients, especially those that can stand soon after trauma. Indications for surgery include damage to the weight-bearing axis, the presence of severe pain, inability to ambulate, significant narrowing of the pelvic canal, neurological deficits, severity of SI displacement and bilateralism and the presence of other injuries affecting ambulation.2, 5-8
Surgical techniques for the stabilisation of SI luxation in cats include lateral SI lag screw placement,9 SI pinning with tension band across the iliac wings,10 trans-sacral screw placement across the iliac wings and sacrum for bilateral SI luxation, with or without nut stabilisation.11-13 Trans-iliac pin stabilisation of SI luxation has also been described as the sole method of stabilisation in cats by Yap et al.,14 in which a pin is passed through the iliac wing either caudal to or through the dorsal spinal process of L7 (Figure 1), and as a bilateral fixation method using a single trans-iliosacral pin by Parslow and Simpson.15 Trans-iliac pins have also been utilised as an indirect method of contralateral stabilisation when combined with screw fixation and trans-sacral pins on the ipsilateral side.16

Placement of lag screws through the ilium has been well described for both the canine and feline sacrum, including the anatomical differences, allowing for reliable and accurate screw positioning.3, 4, 17-19 The criteria for screw fixation in lag fashion for SI luxation repair were listed by Decamp and Braden, and these criteria are now used as a guide for the initial positioning of the screw.20 Shales and Langley-Hobbs17 suggested a ±4° margin of error for both the experimental measurements and operator error when driving screws into the sacrum at an angle specified but estimated 'by eye' of the surgeon. SI lag screw placement can be technically challenging and potentially high risk, leading to many specialists preferring intraoperative imaging and novel instruments to improve their accuracy.19, 21, 22
Trans-iliac pin placement, however, requires a less extensive surgical approach and fewer orthopaedic tools and has a lower risk of damaging the caudal sacral plexus or penetrating the intervertebral canal due to the smaller diameter of the pin. A trans-iliac pin may be an alternative to SI lag screws; however, a safe corridor for this type of implant has not been defined before.
The aim of this study was to identify whether a theoretical predictable safe corridor (with safe avoidance of the spinal canal) is available in cats for trans-iliac pin placement without the use of fluoroscopy and whether this possible corridor was influenced by body weight. This study also evaluated the effect of the start point on the iliac wing for the safety of the pin placement.
Materials and methods
Radiographic measurements
The radiographic database was searched for cats that had pelvic radiographs including straight ventrodorsal (VD) and mediolateral (ML) views of the pelvis (i.e. with superimposition of both iliac wings and acetabulae on the ML views and even-sized obturator foraminae on the VD views). Radiographs were excluded if there was evidence of pelvic fractures. The case records for the cats identified were then searched using the electronic database, and signalment data including age, breed, gender and weight were recorded (Microsoft Excel, Microsoft Corp and SPSS v 19.0 IBM Corp).
The following radiographic measurements were made:
- Pelvic length: measured from the most cranial extent of the ilium to the most caudal extent of the ischiatic tuberosity on the VD view
- Pelvic width: measured between the most lateral extent of the iliac cortices of each hemipelvis at the level of the most cranial margin of the sacrum (S1) on the VD view
- Dorsal lamina of vertebral canal to cranial dorsal iliac spine (DLVC-I): the distance from the dorsal iliac spine to a line superimposed over the dorsal lamina on the ML view (Figure 2)
- Iliac wing area (IWA). A perimeter line drawn around the ilium dorsal to the dorsal vertebral lamina using a custom free-hand drawing tool that calculates the area contained within the drawn perimeter. (Figure 3)

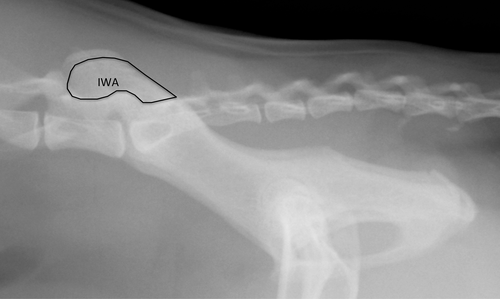
Radiographic evaluation was performed using DICOM imaging software (Osirix version 4.1 64-bit open-source DICOM viewer; Osirix Imaging Software, http://www.osirix-viewer.com/OsiriX-64bit.html).
Defining a hypothetical placement corridor
A trigonometric method was developed to determine the potential deviation of a pin driven from a start point on one iliac wing to its potential exit on the contralateral iliac wing using the measured pelvic width, in one plane and one direction (r), considering a margin of error of ±4 degrees from the perpendicular (Figure 4). As the pin can deviate in any direction, the potential dorsoventral placement corridor is represented by the diameter of the hypothetical placement corridor, and the projection of this cone on the contralateral iliac wing is a semicircle of radius r. The diameter of this semicircle was termed the diameter of the hypothetic placement corridor (dHPC) (Figure 4).
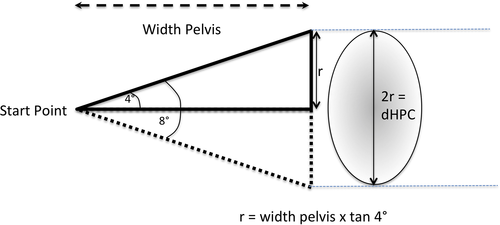
dHPC/DVLC-I comparison
It was hypothesised that a DLVC-I greater than the dHPC should allow safe pin placement without iatrogenic damage to the vertebral canal or its contents. However, the dHPC will be influenced by the start position of the pin on the iliac wing and the diameter of the pin used. Two potential start points were evaluated: first, a midpoint between the distance of the dorsal lamina of the vertebral canal and the caudodorsal iliac spine of the iliac wing and second, a more dorsal approach with the midpoint 2 mm ventral to the most dorsal aspect of the iliac wing. To account for the diameter of the pin used, the diameter of the pin (dpin) was added to the dHPC (d) to give the maximum excursion in one plane in which the pin could exit on the contralateral iliac wing (d + dpin). A range of common cat-appropriate pin sizes was evaluated: 1.2, 1.6 and 2.0 mm pins (Figure 5).
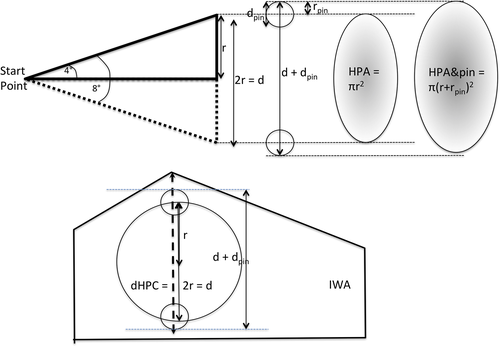
The relationship between dHPC and the DLVC-I was evaluated for both start points with each pin size. In patients where dHPC combined with the pin diameter measure (dHPC+pin) was greater than the DLVC-I, they were termed 'out of range', indicating the danger of iatrogenic damage. If the DLVC-I was greater than dHPC+pin by only 0.2 mm, patients were termed 'borderline'.
Hypothetical cross-sectional area (HPA)/IWA comparison
The HPA (Figure 5) was calculated by comparing the dHPC to the IWA measurement. The area where a pin could potentially exit was calculated using the dHPC and the diameters of pins of 1.2, 1.6 and 2.0 mm (d + dpin) (Figure 5).
Repeatability
Of the 21 cats in the study, 6 were chosen randomly to perform a measurement repeatability test. Radiographs were unlabelled to the operator, and a repeated set of measurements was taken for each cat on different days. The measurements described were repeated five times, with the operator blinded to the previous reading to avoid operator bias. The results were recorded on a spreadsheet (Microsoft excel). All the data were analysed using IBM SPSS Statistic Data Editor, and a mean difference between measurements of <0.5 mm with a range of 1 mm was considered acceptable intra-operator variability.
Statistical analysis
Linear regression analysis was used to assess the association between weight, VD width, VD length, DLVC-I and IWA and whether pelvic length, width or body weight was associated with DLVC-I. The r2 values were calculated, and P < 0.05 was considered significant. A Shapiro–Wilk test was conducted to assess the normality of continuous variables. A P value of <0.05 was considered significant.
Results
Placement corridor
Twenty-one cats with VD and lateral-view straight radiographs without pelvic fractures were identified. Ten cats were neutered males, and 11 cats were neutered females. Domestic Short hair was the most common breed (11/21), followed by Domestic Long hair (3/21); Burmese (2/21); Persian (2/21); and one of each of Russian Blue, Bengal and British Short hair. Mean weight was 4.17 ± 0.89 kg (range 2.0–5.5 kg). All variables were normally distributed (P > 0.05). Mean DLVC-I was 7.53 ± 1.2 mm, and mean pelvic width was 34.36 ± 2.7 mm. The mean dHPC was 4.81 ± 0.38 mm, and mean HPA was 18.25 ± 2.87 mm2. The mean dHPC and HPA values when using pins of diameters of 1.2, 1.6 and 2 mm are summarised in Table 1. The mean IWA was 126.30 ± 22.13 mm2 (Table 1). Overall, all cats evaluated had adequate total area for safe placement of all different sizes of pins.
| DLVC-I | dHPC | dHPC + 1.2 mm pin | dHPC + 1.6 mm pin | dHPC + 2.0 mm pin | |
|---|---|---|---|---|---|
| Mean | 7.53 | 4.81 | 6.01 | 6.41 | 6.81 |
| Min | 5.50 | 3.97 | 5.17 | 5.57 | 5.97 |
| Max | 9.40 | 5.71 | 6.91 | 7.31 | 7.71 |
| SD | 1.20 | 0.38 | 0.38 | 0.38 | 0.38 |
| HPA | HPA + 1.2 mm pin | HPA + 1.6 mm pin | HPA + 2.0 mm pin | IWA | |
|---|---|---|---|---|---|
| Mean | 18.25 | 28.43 | 32.33 | 36.48 | 126.30 |
| Min | 12.39 | 21.01 | 24.38 | 28.01 | 92.90 |
| Max | 25.57 | 37.46 | 41.92 | 46.64 | 180.10 |
| SD | 2.87 | 3.58 | 3.82 | 4.06 | 22.13 |
- dHPC, hypothetic placement corridor diameter; DLVC-I, dorsal lamina of vertebral canal to iliac spine; HPA, hypothetical cross-sectional area; IWA, iliac wing area.
Safe corridors using a mid-iliac wing start point
The mid-iliac wing start point was positioned at a mean distance of 3.76 ± 0.6 mm ventral to the dorsal aspect of the cranial dorsal iliac wing. The remaining DLVC-I after dHPC was taken into account was 2.73 ± 1.2 mm, and hence, all cats had an adequate DLVC-I. However, the available DLVC-I after dHPC combined with a 1.2-mm pin was 1.53 ± 1.2 mm, with 1 of 21 cats having inadequate DLVC-I (out of range) and 3 of 21 cats having marginal DLVC-I (<0.2 mm, borderline). The remaining DLVC-I after dHPC combined with a 1.6-mm pin was taken into account was 1.13 ± 1.2 mm, with 5 of 21 cats having inadequate DLVC-I and 1 of 21 cats having marginal DLVC-I (<0.2 mm, borderline). The available DLVC-I after dHPC combined with a 2.0-mm pin was 0.73 ± 1.2 mm, with 6 of 21 cats having inadequate DLVC-I and 3 of 21 cats having marginal DLVC-I (<0.2 mm, borderline) (Table 2).
| (a) Middle starting point | ||||
|---|---|---|---|---|
| (DLVC-I) − dHPC | (DLVC-I) − dHPC + 1.2 mm pin | (DLVC-I) − dHPC + 1.6 mm pin | (DLVC-I) − dHPC + 2.0 mm pin | |
| Mean | 2.73 | 1.53 | 1.13 | 0.73 |
| Min | 0.77 | −0.43 | −0.83 | −1.23 |
| Max | 4.57 | 3.37 | 2.97 | 2.57 |
| Out of range | 0/21 | 1/21 | 5/21 | 6/21 |
| Borderline | 0/21 | 3/21 | 1/21 | 3/21 |
| (b) Dorsal starting point | ||||
|---|---|---|---|---|
| (DLVC-I) − 2 − dHPC | (DLVC-I) − 2 − (dHPC + 1.2 mm pin) | (DLVC-I) − 2 − (dHPC + 1.6 mm pin) | (DLVC-I) − 2 − (dHPC + 2.0 mm pin) | |
| Mean | 3.21 | 2.61 | 2.41 | 2.21 |
| Min | 1.14 | 0.54 | 0.34 | 0.14 |
| Max | 4.98 | 4.38 | 4.18 | 3.98 |
| Out of range | 0/21 | 0/21 | 0/21 | 0/21 |
| Borderline | 0/21 | 0/21 | 0/21 | 1/21 |
- dHPC, hypothetic placement corridor diameter; DLVC-I, dorsal lamina of vertebral canal to iliac spine.
Safe corridors using a dorsal-iliac wing start point
The dorsal start point was considered to be 2 mm ventral to the most dorsal aspect of the iliac wing. The remaining DLVC-I after dHPC was taken into account was 3.21 ± 1.19 mm. The available DLVC-I after dHPC combined with a 1.2-mm pin was taken into account was 2.61 ± 1.19 mm and 2.41 ± 1.19 mm for the 1.6-mm pin; hence, both pin sizes were considered safe. The remaining DLVC-I after dHPC with a 2.0-mm pin was taken into account was 2.21 ± 1.19 mm; hence, all cats had adequate DLVC-I; however, 1 of 21 cats had marginal DLVC-I (<0.2 mm, borderline) (Table 2).
Repeatability
Repeatability testing showed a mean difference of <0.5 mm for the VD length, VD width, DLVC-I and lateral L7 length. The range of difference in all measurement was 0.5–0.8 mm. Therefore, the intra-operator variability was considered acceptable (Table 3).
| N | Range | Minimum | Maximum | Mean | St. deviation | Variance | |
|---|---|---|---|---|---|---|---|
| VD length | 24 | 0.800 | 0.000 | 0.800 | 0.217 | 0.179 | 0.032 |
| VD width | 24 | 0.600 | 0.000 | 0.600 | 0.179 | 0.169 | 0.029 |
| DLVC-I | 24 | 0.500 | 0.000 | 0.500 | 0.133 | 0.117 | 0.014 |
| Lat L7 length | 24 | 0.700 | 0.000 | 0.700 | 0.213 | 0.168 | 0.028 |
- DLVC-I, dorsal lamina of vertebral canal to iliac spine; Lat, lateral; VD, ventrodorsal.
Linear regression
Table 4 summarises the linear regression analysis between weight, VD width, VD length, DLVC-I and IWA. There was no significant difference between weight, VD width (P = 0.27) and DLVC-I (P = 0.085). Furthermore, weight only accounted for 8% of the variation of VD width in the 21 assessed cats. On the other hand, there was no significant difference between VD width and DLVC-I (P = 0.512); VD width only accounted for 2.3% of the variation in the DLVC-I measurements. Other possible factors contributing to this variation, that is, the space and shape of pelvis and not related to pelvic width, were not assessed in this study.
| VD width | DLVC-I | Weight | ||||
|---|---|---|---|---|---|---|
| r2 | P value | r2 | P value | r2 | P value | |
| Weight | 0.080 | 0.270 | 0.184 | 0.085 | ||
| Area | 0.303 | 0.010 | 0.472 | 0.001 | 0.002 | 0.878 |
| VD width | 0.023 | 0.512 | 0.080 | 0.270 | ||
| VD length | 0.163 | 0.070 | 0.001 | 0.876 | 0.150 | 0.125 |
- DLVC-I, dorsal lamina of vertebral canal to iliac spine; VD, ventrodorsal.
Discussion
The aim of this study was to develop guidelines for placing trans-iliac pins, particularly in the primary care setting. This study demonstrated that the normal feline anatomy does provide a predictable corridor for pin placement. The trigonometric method developed has proved to be useful to estimate two possible start points (mid-iliac and dorsal) for trans-iliac pin placement in cats, although a dorsal start point seemed preferable for a wider pin size. The low intra-observer variability of this method, regardless of intra-operator variability, has shown it to be a valid method, particularly where a fluoroscopy-guided surgery cannot be performed. Furthermore, the lack of significant difference between the patient's size make the guidelines applicable to the standard-size feline breeds.
Using the trigonometric method reported in this study, the hypothetical placement corridor (dHPC) was calculated considering all potential deviations of a pin driven from a defined start point. dHPC results showed that the IWA was large enough to accommodate the dHPC. However, the dHPC is a mathematical circle, and the IWA is not; therefore, the available space in the IWA does not necessarily correlate with the dHPC. This should be considered in the clinical setting when placing a trans-iliac pin, and a certain degree of surgical skill and anatomical navigation is required in order to prevent penetration of the vertebral canal. To this end, the most important consideration is the ventral direction of the diameter of the hypothetical placement corridor (dHPC). The horizontal plane is not clinically relevant as a cranial deviation would only encounter the dorsal spinous process of L7, a caudal deviation would be uneventful, and dorsal deviation would simply miss the contralateral iliac wing. To ensure the correct placement of the pin, the distance from the DLVC-I has to be considered a reference landmark as it delimits the maximum dorsoventral area that can safely accommodate the dHPC. Previous studies have reported the same landmark when defining safe corridors for lag screw placement in cats.3, 19 Burger et al.3 described the sacral tuber as the reference for drilling the screw hole. The sacral tuber is straightforward to visualise intraoperatively and is delimited by the cranial dorsal iliac spine and the caudal dorsal iliac spine. The safest drill point was considered at 70% of the total sacral tuber length when measuring from the cranial dorsal iliac spine. They also reported the ventral gluteal line as an additional landmark, which can also be useful when choosing the pin placement start point. 3, 19
The distance between the DLVC-I is at its greatest at the most craniodorsal aspect of the iliac wing. Identifying this landmark guarantees the largest dorsoventral area to place the pin within the dHPC and avoids the vertebral canal ventrally. The mid-way start point situates the dHPC limits closer to the dorsal lamina of the vertebral canal; if then the additional space occupied by the pin is taken into account (Figure 5), the margin of error is reduced considerably. When testing the proposed mid-way start point in different radiographs, 1 of 21 cats had inadequate DLVC-I measurement and 3 of 21 cats had marginal measurement with the small-diameter pin (1.2 mm). The number of cats with inadequate or marginal DLVC-I measurement increased to 6 of 21 and 3 of 21 cats, respectively, when increasing the pin diameter. Therefore, in some cats, larger pins may deviate out of the safe corridor. Careful consideration should be given to the selection of a suitable pin that will provide stability but minimise malpositioning. A 1.2-mm pin would be the safest option if considering a mid-iliac start point; however, this pin would be weaker than a larger pin. Currently, the biomechanics of a trans-iliac pin construct are poorly understood, and biomechanical or finite element studies are not available; hence, surgeons may prefer to use a larger pin, which could be achieved when using the dorsal start point. The dorsal start point could accommodate the 1.6-mm and 2.0-mm trans-iliac pin safely, and only one cat had marginal measurement (<0.2 mm). Therefore, theoretically, the greater the dorsal start point, the more room present to accommodate various sizes of the trans-iliac pin up to 2.0 mm while reducing the chance of iatrogenic nerve damage. For the purpose of this study, the dorsal starting point was established 2 mm ventral to the most dorsal aspect of the iliac wing as it was considered the most appropriate location with regard to dorsal and medial bone stock. However, no biomechanical studies have tested the adequacy of this location to prevent pin pull-out other than anecdotal surgical experience. On balance, the authors' clinical preference is to use a dorsal starting pin with a 1.6-mm pin as it probably offers the best trade-off in terms of placement safety and biomechanical stabilisation.
The strict inclusion criteria for this study limited the number of suitable radiographs due to the requirement for good superimposition of the iliac wings and acetabula. The 21 cases included were of varying breeds and weights, giving a sample of the authors' institution feline population. The repeatability for the measurements taken in this study was found to be at an acceptable level as illustrated in Table 3. The regression analysis in Table 4 did not show a significant relationship between weight and VD width (P = 0.27, r2 = 0.08), weight and VD length (P = 0.12, r2 = 0.15), weight and DLVC-I (P = 0.184, r2 = 0.085) and VD width and DLVC-I. (P = 0.512, r2 = 0.023). This implies that increasing body weight does not affect the pelvic width or length. Therefore, the guidelines for safe pin placement developed here should be largely appropriate for most cats seen clinically, which are commonly between 2.0 and 5.5 kg.
Trans SI lag screw fixation for SI luxation is currently the surgical method of choice in cats.20 However, the safe drill corridor is narrow at 0.5 cm2 or less, and the drilling angles can be difficult to achieve.3 One study showed that 12.5% of lag screws placed into the sacrum of 40 cats were found to be malpositioned, which can result in cauda equina injury, damage of the median sacral vessels and/or increase risk of screw loosening if the screw is less than 60% depth of the sacral width.9 In comparison, trans-iliac pinning may be safer as the pin is positioned further away from major neurovascular structures. It has also been reported that adjusting implant direction is relatively easier due to the wider safe corridor and that sciatic and femoral nerve injuries are lower as no sacral manipulation is required.10, 23 Another potential advantage is the shorter anaesthetic time required for placement.14, 15 Although an SI lag screw has shown increased friction in the SI joint, providing a more stable construct, Yap et al.14 hypothesised that iliac wing compression achieved by trans-iliac implants is enough to reduce hemipelvis displacement based on their reported clinical cases. 14, 15, 24 Trans-iliac pinning, however, is not without risks, with pin loosening and/or soft tissue focal irritation reported in dogs 4–6 weeks postoperatively.24 Trans-iliac pin bending has also been reported in one cat by Yap et al.14 Overall, clinical reports appear to support trans-iliac pinning as an alternative to trans-iliosacral lag screw. However, it has to be acknowledged that a trans-iliac pin is likely to be less mechanically stable than an SI lag screw, which is placed through the joint itself, as the trans-iliac pin is eccentrically positioned to the joint, although the amount of stability required is unknown as healing is through fibrosis and therefore may not require the same stability as bone fracture healing.24
This study used a trigonometric method of determining the safe corridor using plain radiographs as they are widely available in the primary care setting. It is worth noting that the radiographic measures used here provide an effective safety margin as the assumptions do not account for the fact that the neural canal is oval in cross section and not the full width of the sacrum. Alternatively, computed tomography could be used to provide a more accurate assessment of the safe corridor available, particularly if a larger pin diameter is biomechanically warranted.
This study demonstrates that the normal anatomy of a cat gives a relatively predictable window for pin placement. It also provides guidelines for positioning different sizes of pins to avoid spinal canal penetration. In the clinical setting, this method would be more appropriate for the management of unilateral SI luxations in cats as the landmarks described are applied to only one iliac wing. During surgery for unilateral SI luxations, from a bilateral dorsal approach to the iliac wing, it is advisable to align the luxated contralateral wing through traction and then compress the wing against the sacrum using large pointed reduction forceps. The points are placed from one iliac wing to the other on the iliac fossa ventral to the proposed pin starting point, which helps resist wing displacement as the trans-iliac pin is placed. Malposition of a unilaterally luxated ilium should only result in the pin missing the dorsal aspect of the luxated reduced ilium and would not therefore impact the safety factors identified here as long as the pin is started from the non-luxated side. For bilateral luxations, an SI lag screw could be placed unilaterally, and a trans-iliac pin could follow. Parslow and Simpson15 described a stabilisation technique using trans-iliosacral pinning for bilateral SI luxation in cats that could be an alternative to the trigonometric method described in this article. Pratesi et al.13 also proposed a single screw placement to stabilise bilateral luxations, which may be simpler, have a lower risk and technical difficult than placing bilateral SI lag screws, although both Parslow and Simpson15 and Pratesi et al.13 require a C-shaped aiming device. Clearly, if an SI screw was placed unilaterally followed by a trans-iliac pin to facilitate stability of the contralateral luxation, the theoretical safe corridor would be entirely dependent on a highly accurate reduction of the lag screw-stabilised SI fixation side, which is not always achieved. As the safety of trans-iliac pin placement in bilateral SI luxation was not directly assessed in our predictive model, it is not routinely recommended here.
Other stabilisation techniques could also be considered; however, in the authors' opinion, the skills required and the risks involved in trans-iliac pin placement are less than lag screw fixation in the sacrum. This is due to the larger area for implant placement and the more distant relationship of the pin tract to the vertebral canal and its nervous tissue, and hence, this technique may be useful for clinicians with less experience in placing SI screws or when equipment or referral are not available. Likewise, a trans-iliac pin could be placed as an adjunct to an SI lag screw where increased stability was desirable.
Conclusions
All cats in this study had a definable safe corridor available for trans-iliac pin placement; however, ensuring the correct start point was critical. Although a 1.2-mm pin is recommended for midway DLVC-I trans-iliac pin placement to avoid vertebral penetration, the authors do not advocate its use due to the unproven but expected relatively weak mechanical support provided. A dorsal start point of 2 mm ventral to the most dorsal point of the iliac wing can accommodate the maximum recommended 2-mm pin provided the iliac wings are aligned, although 1.6 mm may be safer. Overall, the surgeon should utilise the smallest pin they can afford without compromising the stability of the construct in order to reduce the likelihood of vertebral canal penetration in simple unilateral SI luxations.
Conflicts of interest and sources of funding
The authors declare no conflicts of interest or sources of funding for the work presented here.




