Effects of quercetagetin on the growth performance, nutrient digestibility, slaughter performance, meat quality, and antioxidant capacity of broiler chickens
Abstract
This study investigated the effects of quercetagetin (QG) on growth performance, nutrient digestibility, meat quality, and antioxidant capacity of broilers. Four hundred 1-day-old Cobb broilers were randomly divided into five diets, each with eight replicates and 10 birds per replicate. The diets included a basal diet, and four diets with 25, 50, 100, and 200 mg/kg QG supplemented in basal diet. Body weight on d 21, average daily gain, and average daily feed intake on days 1–21 were quadratically (p < 0.05) increased with increasing QG supplementation. The apparent digestibility of crude protein, ether extract, and total phosphorus increased linearly (p < 0.05) from day 1 to 21, and increased quadratically (p < 0.05) from day 22 to 42. The L* values of leg muscles were lower (p < 0.05) in QG groups than control group. QG supplementation quadratically (p < 0.05) elevated glutathione peroxidase (GSH-Px) activity and reduced malondialdehyde (MDA) levels in serum. The L* value was negatively correlated with GSH-Px. These results suggested that QG supplementation (50–100 mg/kg) enhanced early growth, nutrient digestibility, and antioxidant status in broilers, highlighting its functional properties and potential as an additive to improve broiler productivity.
1 INTRODUCTION
Intensive modern farming gives a boost to livestock productivity and economic benefits. However, the problems associated with intensive feeding are causing increasing concern. Broilers suffer from an excess of reactive oxygen radicals due to many factors, such as feeding environment, density, feed quality, and transportation, which imbalance the oxidative and antioxidant systems of the body and cause oxidative stress (Allen et al., 2023; Damiano et al., 2022; Nasr et al., 2021; Nawaz et al., 2021). Oxidative stress causes lipid peroxidation and protein denaturation in broiler chickens, resulting in functional damage to the liver, intestine, and muscle tissues, affecting poultry health and product quality, and severely affecting the efficient production and sustainable development of the poultry industry (Chen et al., 2021). Therefore, it is vital to find a natural green feed additive that can improve stress resistance potential and reduce oxidative stress.
Flavonoids are one of the major secondary metabolites of plants, including flavones, flavonols, flavanones, flavanols, anthocyanins, and isoflavones (Panche et al., 2016). Studies have shown that flavonoids have various biological functions, such as antioxidant, anti-inflammatory, and antiviral functions, and improve the redox status of livestock (Ahmed et al., 2022; Bachmetov et al., 2012; Tang et al., 2019). Marigold (Tagetes erecta L.) is a natural forage plant and an annual herb of the genus Tagetes in the Asteraceae family (Zhang et al., 2019). There were four antioxidant components in marigolds, including quercetagetin-7-O-glucoside, quercetagetin (C15H10O8, QG), quercetin, and patulin. Among these, QG was more capable of scavenging DDPH-free radicals (Huang et al., 2022).
QG, as a new feed additive, is chemically identified as 3,3,4,5,6,7-hexahydroxyflavone, belongs to the flavonols (Xu et al., 2011), which has one more hydroxyl group than traditional quercetin and exhibit stronger biological activity and great potential for application as an additive. Some studies have found that quercetin can scavenge free radicals and improve broiler production performance, meat quality, and antioxidant capacity (Dong et al., 2020; Sun et al., 2020). However, there are few reports on the effects of QG on broiler chickens, and the effective dosage is inconsistent. In order to understand the safety and efficacy of QG on broilers, we determined the effects of different doses of QG supplementation on the growth performance, apparent digestibility of nutrients, slaughter performance, meat quality, and antioxidant capacity of broiler chickens. The results of this study will provide a reference for the application of QG in broiler production.
2 MATERIALS AND METHODS
2.1 Animal welfare statement
All animal experiments were conducted according to the ARRIVE guidelines and were carried out on the basis of the UK Animals (Scientific Procedures) Act, 1986, and associated guidelines, as well as the EU Directive 2010/63/EU for animal experiments. All experimental procedures were approved by the Animal Care and Use Committee of Hebei Agricultural University (Baoding, China, protocol number: 2023140).
2.2 Materials
QG is a hexahydroxy flavonoid extracted from the residue of marigold after the removal of lutein (Figure 1). Its purity exceeded 80% and was provided by Chenguang Co., Ltd. (Handan, China).
2.3 Experimental design, animal, and management
Four hundred healthy 1-day-old Cobb broiler chickens (half male and half female) were selected and randomly divided into five treatments with eight replicates (10 birds per replicate). The control group was fed the basal diet, and the experimental group was fed the basal diet supplemented with 25, 50, 100, and 200 mg/kg QG. The experimental period was 42 days. The basal diets of the starter (1–21 days) and grower phases (22–42 days) were formulated according to the Chinese feeding standard for chickens (NY/T 33–2004; The Ministry of Agriculture of the People's Republic of China, 2004), and the composition and nutritional levels of the basal diet are shown in Table 1. Dry matter, calcium, and total phosphorus were determined according to AOAC standards (AOAC, 2006). Total nitrogen was measured as determined by the Kjeldahl method, and crude protein (CP) was calculated by Factor N × 6.25. Ether extract was measured using a Soxhlet extractor. The amino acid composition was analyzed using ultra-performance liquid chromatography (UPLC). The coop temperature was set at 32°C from day 1 to day 14, then gradually reduced until it reached 25°C and was maintained until day 42. The average relative humidity was between 60 and 75%, and the birds were provided with continuous fluorescent lighting according to the lighting program outlined in the Cobb Management Guide (Cobb-Vantress, 2018). Feed and drinking water were supplied freely to all experimental groups throughout the experiment.
| Items | Trial period | |
|---|---|---|
| Starter phase (1–21 days) | Grower phase (22–42 days) | |
| Ingredients | ||
| Corn | 48.91 | 57.18 |
| Soybean meal | 42.40 | 35.10 |
| Soybean oil | 5.10 | 4.50 |
| Stone powder | 0.99 | 1.06 |
| Calcium hydrogen phosphate | 1.49 | 1.05 |
| Salt | 0.30 | 0.30 |
| L-Lysine hydrochloride | 0.15 | 0.17 |
| DL-Methionine | 0.16 | 0.14 |
| Premixesa | 0.50 | 0.50 |
| Total | 100.00 | 100.00 |
| Nutrient levels | ||
| Dry matter | 89.78 | 89.53 |
| Metabolic energy (kcal/kg)b | 3050.00 | 3100.00 |
| Crude protein | 21.38 | 20.37 |
| Calcium | 0.85 | 0.75 |
| Total phosphorus | 0.65 | 0.55 |
| Lysine | 1.35 | 1.20 |
| Methionine | 0.51 | 0.47 |
| Threonine | 0.88 | 0.78 |
| Tryptophan | 0.29 | 0.25 |
| Valine | 1.08 | 0.96 |
- a The premix provided the following per kg of diet: vitamin A,10,000 IU; vitamin D3, 5,000 IU; vitamin E, 80 IU; vitamin K3, 2.0 mg; vitamin B1, 3.2 mg; vitamin B2, 8.5 mg; vitamin B6, 5 mg; vitamin B12, 0.02 mg; biotin, 0.2 mg; folic acid, 1.6 mg; pantothenic acid, 15 mg; niacin, 50 mg; Cu, 10 mg; Fe, 50 mg; Mn, 120 mg; Zn, 100 mg; I, 1.25 mg; Se, 0.3 mg; choline chloride, 1500 mg.
- b ME was a calculated value, and the other nutrient levels were measured values.
2.4 Growth performance
The body weight (BW) was measured on day 1, day 21, and day 42 of the experiment after a 12-h period of feed deprivation to calculate the average daily gain (ADG). Feed consumption was recorded weekly to calculate the average daily feed intake (ADFI). Feed-to-gain ratio (F/G) was calculated as follows: (F/G) = total feed consumption/total BW gain.
2.5 Nutrient digestibility
The nutrient digestibility of broiler chickens in each group was measured using the endogenous indicator method (acid insoluble ash, AIA). Fresh feces were collected from each replicate continuously for 5 days before the end of the experimental period. Particles such as feathers, feed, or dust were carefully removed from the feces. Fecal samples used for crude protein analysis required nitrogen fixation with 10% hydrochloric acid. Other fecal samples were dried and smashed for general component analysis according to the method of AOAC (2006).
2.6 Slaughter performance
At the end of the experiment, each broiler chicken was fasted for 12 h. The broiler with a BW close to the average weight of each replicate cage was selected, euthanized by cervical dislocation, and manually dissected by trained personnel to measure the weight of the carcass, breast muscle, leg muscle, and abdominal fat. The dressed percentage was calculated relative to live BW, while other rates were calculated as percentages of carcass weight (Zhu et al., 2022).
2.7 Meat quality
2.7.1 Meat color
An Iwave W R-18 precision colorimeter was used to measure meat color, including a*, b*, and L* values at 1 point from each of the upper, middle, and lower parts of the meat samples, and finally the average value was calculated.
2.7.2 pH
The pH values were measured at 45 min and 24 h after slaughter using a Testo-205 pH meter at 1 point from each of the top, middle, and bottom sections of the muscle sample, and then the average value was calculated.
2.7.3 Cooking loss
Cooking loss was measured as follows: take 5–10 g of meat samples that have been refrigerated for 24 h in the sample bag, place them in a constant temperature water bath at 80°C, and keep heating until the center of the muscle reaches 70°C. Then, cool the muscles to 0–4°C and weigh them to calculate the cooking loss.
2.7.4 Shear force
Shear force was determined by placing the meat samples in plastic bags in a water bath at a temperature of 75–80°C for 2 h and then cooling under running water for 30 min. Meat samples with length, width, and thickness of 1.5, 1.0, and 0.5 cm were intercepted in a direction parallel to the muscle fibers, and shear force was measured using a CLM-3B muscle tenderness meter.
2.7.5 Drip loss
A 5 cm × 3 cm × 2 cm meat sample was taken, weighed, hung in the refrigerator at 4°C, and weighed again after 24 h to calculate drip loss.
2.8 pH of intestinal digesta
At the end of the trial, broiler chickens with a BW close to the average weight of each replicate cage were chosen. The digesta of the duodenum, jejunum, ileum, and cecum were quickly removed, and pH values were recorded at three different points using a Testo-205 pH meter.
2.9 Organ index
The heart, liver, spleen, thymus, and bursa of broiler chickens were excised and weighed for organ index analysis. Organ index = organ weight/live weight.
2.10 Serum antioxidant
At the end of the experiment, broiler chickens were fasted for 12 h. One broiler from each replicate cage was randomly selected to collect blood (10 mL) from the wing vein. Blood samples were collected in sterile procoagulant tubes, centrifuged at 3000 rpm/min for 10 min to obtain serum, and stored at −20°C. The activities of total antioxidant capacity (T-AOC, catalog number: YX-200118C), glutathione peroxidase (GSH-Px, catalog number: YX-071908C), superoxide dismutase (SOD, catalog number: YX-191504C), and peroxidase (CAT, catalog number: YX-030120C), as well as malondialdehyde (MDA, catalog number: YX-130401C) level were determined using commercial kits (Beijing Bo Rui Long Term Technology Co Ltd., China) and measured according to the manufacturer's instructions. A Spearman correlation analysis was performed to evaluate the relationship between serum antioxidant indexes and meat quality.
2.11 Statistical analysis
The normality of data among groups was confirmed and screened using the UNI-VARIATE procedure of SAS V 9.4 (SAS Inst. Inc., Cary, NC, USA). The data were analyzed using the general linear model (GLM) procedure of SAS. Diets were used as sources of variation, and the individual bird from each replicate cage was used as the experimental unit except for the growth performance data, which used a replicate cage as the experimental unit. Data were expressed as the least-squares means (LSMean) and pooled standard errors of the mean value (SEM). Differences among treatments were determined by Tukey's multiple range tests. Contrast statements were used to analyze the linear and quadratic effects of the inclusion level of QG. p < 0.05 was considered a significant difference.
3 RESULTS
3.1 Effects of quercetagetin on the growth performance of broiler chickens
To evaluate the dose–response effects of increasing supplement levels of QG on the growth performance of broiler chickens, linear and quadratic analyses were performed for different doses of QG groups (Table 2). On day 1, the BW of broiler chickens was not different among groups (p > 0.05). On day 21, boiler chickens fed diets supplemented with 100 mg/kg QG had a higher BW than the control group (p < 0.05). Additionally, the BW of broiler chickens on day 21 increased quadratically with an increasing dose of QG supplementation (p < 0.05). From day 1 to day 21, the ADG was higher in the 50 and 100 mg/kg QG groups than in the control group (p < 0.05), and the ADG and ADFI increased quadratically (p < 0.05) with increasing dose of QG supplementation. From day 22 to day 42 and day 1 to day 42, there were no differences in ADG, ADFI, or F/G in each group (p > 0.05).
| Items | Control | Quercetagetin levels (mg/kg) | SEM | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | Trt. | L | Q | |||
| Day 1 BW (g) | 40.95 | 41.03 | 40.96 | 40.42 | 40.88 | 0.22 | 0.307 | 0.441 | 0.160 |
| Day 21 BW (g) | 681.80b | 693.93ab | 701.35ab | 710.11a | 694.63ab | 5.79 | 0.041 | 0.192 | 0.004 |
| Day 42 BW (g) | 2525.71 | 2488.04 | 2475.11 | 2463.50 | 2481.31 | 48.03 | 0.913 | 0.606 | 0.432 |
| Starter 1–21 days | |||||||||
| ADG (g/day) | 30.69b | 31.20ab | 31.79a | 31.75a | 30.71b | 0.30 | 0.024 | 0.601 | 0.002 |
| ADFI (g/day) | 41.12b | 42.65ab | 42.91a | 41.88ab | 41.61ab | 0.38 | 0.012 | 0.483 | 0.040 |
| F/G | 1.34 | 1.37 | 1.35 | 1.32 | 1.36 | 0.02 | 0.308 | 0.977 | 0.265 |
| Grower 22–42 days | |||||||||
| ADG (g/day) | 91.68 | 90.37 | 88.33 | 88.30 | 89.78 | 2.34 | 0.824 | 0.644 | 0.290 |
| ADFI (g/day) | 147.52 | 146.33 | 145.02 | 147.75 | 147.08 | 2.32 | 0.921 | 0.843 | 0.841 |
| F/G | 1.62 | 1.62 | 1.64 | 1.68 | 1.64 | 0.03 | 0.707 | 0.466 | 0.267 |
| Whole period 1–42 d | |||||||||
| ADG (g/day) | 61.99 | 60.38 | 59.37 | 59.10 | 59.52 | 1.00 | 0.283 | 0.146 | 0.096 |
| ADFI (g/day) | 94.43 | 93.63 | 92.72 | 93.21 | 93.06 | 1.13 | 0.859 | 0.510 | 0.492 |
| F/G | 1.53 | 1.55 | 1.56 | 1.58 | 1.57 | 0.02 | 0.482 | 0.226 | 0.154 |
- Note: Mean values differ significantly (p < 0.05) within the same row for different letters. Values are LSMean and pooled standard error of the mean values (n = 8).
- Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; F/G, feed intake/weight gain; L, linear; Q, quadratic effects of quercetagetin supplementation dose (0, 25, 50, 100, 200 mg/kg); Trt., treatment.
3.2 Effects of quercetagetin on apparent digestibility of nutrients in broiler chickens
From day 1 to day 21, the apparent total tract digestibility of crude protein and total phosphorus demonstrated a linear (p < 0.05, Table 3) response to increasing levels of QG, with a maximum observed for the birds fed the diet supplemented with 100 mg/kg QG. The apparent digestibility of ether extract increased linearly (p < 0.05) and quadratically (p < 0.05) with increasing QG supplementation. The apparent digestibility of calcium was not different among all groups (p > 0.05). From day 22 to day 42, a quadratic (p < 0.05) response was observed for the digestibility of crude protein, ether extract, and total phosphorus, with a maximum corresponding to the 100 mg/kg QG group. The digestibility of calcium had a trend of linear (p = 0.072) and quadratic (p = 0.088) increase with increasing QG supplementation.
| Items | Control | Quercetagetin levels (mg/kg) | SEM | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | Trt. | L | Q | |||
| 1–21 days | |||||||||
| Crude protein | 52.33b | 54.13ab | 53.73ab | 56.17a | 55.75ab | 0.85 | 0.020 | 0.006 | 0.084 |
| Ether extract | 79.86b | 82.52ab | 82.18ab | 87.32a | 84.54ab | 1.68 | 0.043 | 0.033 | 0.038 |
| Calcium | 61.50 | 62.16 | 56.92 | 64.04 | 61.61 | 2.03 | 0.206 | 0.620 | 0.979 |
| Total phosphorus | 54.04b | 54.21b | 52.66b | 63.35a | 61.33a | 1.25 | < 0.001 | < 0.001 | 0.124 |
| 22–42 days | |||||||||
| Crude protein | 59.44 | 58.13 | 61.77 | 64.90 | 54.43 | 2.42 | 0.121 | 0.303 | 0.021 |
| Ether extract | 89.47 | 89.73 | 90.34 | 91.11 | 88.15 | 0.67 | 0.076 | 0.151 | 0.010 |
| Calcium | 61.75 | 61.62 | 66.83 | 61.96 | 58.46 | 1.81 | 0.058 | 0.072 | 0.088 |
| Total phosphorus | 56.20 | 55.49 | 59.50 | 61.86 | 52.23 | 2.40 | 0.080 | 0.292 | 0.014 |
- Note: Mean values sharing different letters (a, b) differ significantly (p < 0.05). SEM = pooled standard error of the LSMean (n = 8).
- Abbreviations: L, linear; Q, quadratic effects of quercetagetin supplementation dose (0, 25, 50, 100, 200 mg/kg); Trt., treatment.
3.3 Effects of quercetagetin on the slaughter performance of broiler chickens
To assess the effect of different levels of QG on slaughter performance of broiler chickens, we determined dressed percentage, half-eviscerated carcass rate, eviscerated rate, breast and leg muscle rate, and abdominal fat rate. The breast muscle rate increased linearly (p < 0.05) with increasing QG supplementation, and the 50 mg/kg QG group had the maximum. QG supplementation had no effect (p > 0.05, Table 4) on dressed percentage, half-eviscerated and eviscerated rate, leg muscle rate, and abdominal fat percentage of broiler chickens.
| Items | Control | Quercetagetin levels (mg/kg) | SEM | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | Trt. | L | Q | |||
| Dressed percentagea | 89.01 | 90.07 | 88.39 | 90.50 | 90.06 | 0.58 | 0.129 | 0.180 | 0.614 |
| Half-evisceratedb | 82.36 | 83.71 | 81.23 | 83.39 | 82.61 | 1.06 | 0.531 | 0.919 | 0.895 |
| Evisceratedb | 73.08 | 73.43 | 71.45 | 72.44 | 73.13 | 1.18 | 0.770 | 0.945 | 0.417 |
| Breast muscleb | 28.94 | 29.01 | 30.77 | 28.42 | 26.60 | 0.94 | 0.105 | 0.037 | 0.221 |
| Leg muscleb | 19.98 | 19.29 | 20.81 | 20.13 | 20.27 | 0.99 | 0.866 | 0.742 | 0.782 |
| Abdominal fatb | 1.57 | 1.72 | 0.94 | 2.57 | 1.44 | 0.58 | 0.429 | 0.861 | 0.418 |
- Note: Means sharing different letters (a, b) differ significantly (p < 0.05). SEM = pooled standard error of the means (n = 8).
- Abbreviations: L, linear; Q, quadratic effects of quercetagetin supplementation dose (0, 25, 50, 100, 200 mg/kg); Trt., treatment.
- a Relative to live body weight.
- b Relative to carcass weight.
3.4 Effects of quercetagetin on meat quality of broiler chickens
The effect of QG supplementation on the meat quality of breast and leg muscles were also evaluated (Table 5). There was no difference on a* and b* values, pH 45 min, cooking loss, drip loss, or shear force in the breast and leg muscles among all groups (p > 0.05). The lightness value (L*) of breast muscle was lower (p < 0.05) in broiler chickens fed diets with 50 and 200 mg/kg QG compared with the 100 mg/kg group. Diets supplementation with QG reduced the L* value of the leg muscle compared with birds fed the control diet (p < 0.05). The pH 24 h value of leg muscle had a tendency to increase in the 100 mg/kg group compared with the 200 mg/kg group (p = 0.081).
| Items | Control | Quercetagetin levels (mg/kg) | SEM | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | Trt. | L | Q | |||
| Breast muscle | |||||||||
| L* | 40.21ab | 37.71ab | 34.90b | 41.82a | 34.13b | 1.55 | 0.010 | 0.191 | 0.367 |
| a* | 1.63 | 2.60 | 2.57 | 1.62 | 2.51 | 0.31 | 0.057 | 0.486 | 0.790 |
| b* | 6.88 | 7.81 | 5.18 | 6.73 | 7.50 | 0.64 | 0.054 | 0.521 | 0.141 |
| pH 45 min | 6.86 | 6.91 | 6.87 | 6.68 | 6.80 | 0.08 | 0.334 | 0.288 | 0.285 |
| pH 24 h | 6.37 | 6.31 | 6.24 | 6.28 | 6.21 | 0.07 | 0.573 | 0.179 | 0.654 |
| Cooking loss (%) | 27.33 | 26.54 | 27.97 | 28.99 | 27.15 | 1.16 | 0.589 | 0.836 | 0.221 |
| Drip loss (%) | 2.07 | 2.32 | 3.03 | 2.26 | 2.68 | 0.40 | 0.482 | 0.491 | 0.664 |
| Shear force (N) | 15.95 | 23.58 | 21.56 | 17.02 | 25.25 | 4.67 | 0.576 | 0.391 | 0.763 |
| Leg muscle | |||||||||
| L* | 43.60a | 37.20 | 36.92b | 39.08b | 35.94b | 1.30 | 0.004 | 0.009 | 0.184 |
| a* | 4.10 | 5.43 | 7.00 | 5.06 | 6.37 | 0.70 | 0.096 | 0.175 | 0.418 |
| b* | 7.76 | 8.36 | 9.91 | 8.02 | 10.22 | 0.72 | 0.072 | 0.067 | 0.860 |
| pH 45 min | 6.94 | 6.74 | 6.80 | 6.89 | 6.66 | 0.08 | 0.120 | 0.091 | 0.592 |
| pH 24 h | 6.66 | 6.61 | 6.52 | 6.71 | 6.48 | 0.06 | 0.081 | 0.141 | 0.300 |
| Cooking loss (%) | 27.44 | 28.33 | 29.22 | 28.12 | 29.56 | 1.20 | 0.741 | 0.334 | 0.901 |
| Drip loss (%) | 2.13 | 1.75 | 2.79 | 2.29 | 2.83 | 0.63 | 0.665 | 0.372 | 0.939 |
| Shear force (N) | 15.38 | 19.77 | 21.94 | 14.17 | 20.01 | 1.23 | 0.240 | 0.391 | 0.763 |
- Note: Means sharing different letters (a, b) differ significantly (p < 0.05). SEM = pooled standard error of the LSMean (n = 8).
- Abbreviations: L, linear; Q, quadratic effects of quercetagetin supplementation dose (0, 25, 50, 100, 200 mg/kg); Trt., treatment. L*, brightness of meat. a*, redness of meat. b* , yellowness of meat.
3.5 Effects of quercetagetin on the pH of the intestinal digesta of broiler chickens
Since the pH of the intestinal digesta is related to nutrient digestion and absorption, we determined the pH values of the intestinal digesta in different fractions. Compared with the control group, the addition of QG to the diet had no effect on the pH of the duodenal and cecal digesta of broiler chickens (p > 0.05, Table 6). However, the 25 mg/kg QG group had a lower pH value in the jejunal digesta than the 50 mg/kg group (p < 0.05).
| Items | Control | Quercetagetin levels (mg/kg) | SEM | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | Trt. | L | Q | |||
| Duodenum | 6.83 | 6.83 | 6.72 | 6.87 | 6.85 | 0.07 | 0.610 | 0.580 | 0.772 |
| Jejunum | 6.74ab | 6.55b | 6.84a | 6.74ab | 6.64ab | 0.06 | 0.017 | 0.588 | 0.165 |
| Ileum | 6.78 | 6.95 | 7.16 | 7.04 | 6.61 | 0.14 | 0.064 | 0.141 | 0.013 |
| Cecum | 7.12 | 7.26 | 7.27 | 7.05 | 7.30 | 0.09 | 0.265 | 0.469 | 0.464 |
- Note: Means sharing different letters (a, b) differ significantly (p < 0.05). SEM = pooled standard error of the LSMean (n = 8).
- Abbreviations: L, linear; Q, quadratic effects of quercetagetin supplementation dose (0, 25, 50, 100, 200 mg/kg); Trt., treatment.
3.6 Effects of quercetagetin on the organ index of broiler chickens
As shown in Table 7, the addition of QG had no effect on the heart, liver, spleen, and bursa index of broiler chickens compared with the control group (p > 0.05), whereas diets supplementation with 25 mg/kg QG improved the thymus index compared with broiler chickens fed the control diet (p < 0.05).
| Items | Control | The amount of quercetagetin (mg/kg) | SEM | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | Trt. | L | Q | |||
| Heart | 4.34 | 4.30 | 3.79 | 4.59 | 4.36 | 0.22 | 0.134 | 0.502 | 0.903 |
| Liver | 18.38 | 17.56 | 17.59 | 18.55 | 19.22 | 0.52 | 0.169 | 0.053 | 0.376 |
| Spleen | 0.99 | 1.04 | 0.99 | 0.99 | 1.12 | 0.11 | 0.935 | 0.495 | 0.650 |
| Thymus | 2.33b | 4.64a | 2.88ab | 3.30ab | 4.10ab | 0.52 | 0.025 | 0.167 | 0.973 |
| Bursa | 1.32 | 1.67 | 2.02 | 1.67 | 1.59 | 0.19 | 0.146 | 0.772 | 0.092 |
- Note: Means sharing different letters (a, b) differ significantly (p < 0.05). SEM = pooled standard error of the LSMean (n = 8).
- Abbreviations: L, linear; Q, quadratic effects of quercetagetin supplementation dose (0, 25, 50, 100, 200 mg/kg); Trt., treatment.
3.7 Effects of quercetagetin on serum antioxidant activity in broiler chickens
To evaluate whether the observed effects of QG on the performance of broiler chickens were associated with antioxidant function, we determined the impact of diet supplementation with QG on serum antioxidant indexes. As shown in Table 8, the GSH-Px activity was higher in the 50 and 100 mg/kg QG groups than that in the control, 25 and 200 mg/kg QG groups (p < 0.05), as well as, quadratically (p < 0.05) increased with increasing QG supplementation. Compared with the 25 mg/kg QG group, the serum CAT activity in the 50 mg/kg QG group was increased (p < 0.05). The serum MDA levels showed a quadratic decrease (p < 0.05) with increasing QG supplementation. There was no difference (p > 0.05) in SOD and T-AOC activities in the serum of broilers among all groups.
| Items | Control | The amount of quercetagetin (mg/kg) | SEM | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | Trt. | L | Q | |||
| SOD (U/mL) | 274.77 | 281.85 | 265.35 | 272.07 | 265.35 | 18.02 | 0.977 | 0.742 | 0.654 |
| GSH-Px (U/mL) | 434.54b | 433.57b | 476.68a | 478.30a | 436.82b | 6.43 | 0.022 | 0.209 | 0.013 |
| CAT (U/mL) | 88.20ab | 86.69b | 96.17a | 92.26ab | 90.98ab | 1.11 | 0.044 | 0.112 | 0.118 |
| T-AOC (U/mL) | 10.60 | 11.45 | 9.93 | 11.35 | 12.81 | 0.68 | 0.757 | 0.380 | 0.482 |
| MDA (nmol/mL) | 2.15 | 2.02 | 1.96 | 1.72 | 2.23 | 0.06 | 0.082 | 0.757 | 0.043 |
- Note: Means sharing different letters (a, b) differ significantly (p < 0.05). SEM = pooled standard error of the LSMean (n = 8).
- Abbreviations: CAT, peroxidase; GSH-Px, glutathione peroxidase; L, linear; MDA, malondialdehyde; Q, quadratic effects of quercetagetin supplementation dose (0, 25, 50, 100, 200 mg/kg); SOD, superoxide dismutase; T-AOC, total antioxidant capacity; Trt., treatment.
3.8 Correlation analysis between serum antioxidant composition and meat quality
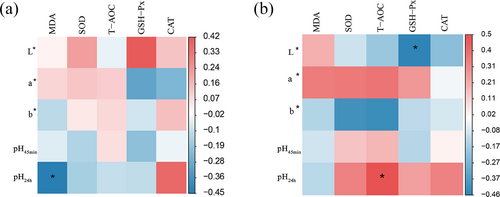
A Spearman correlation analysis was performed to determine the relationship between serum antioxidant capacity and parameters of meat quality. The L* value was negatively correlated with GSH-Px activity in leg muscle. The pH 24 h was negatively correlated with MDA in the breast muscles and positively correlated with T-AOC activity in the leg muscles (Figure 2a,b).
4 DISCUSSION
Many studies have shown that flavonoids promoted the growth performance of broilers (Prihambodo et al., 2021). QG is chemically identified as 3,3,4,5,6,7-hexahydroxyflavone and belongs to the flavonols. It is a polyhydroxyphenol compound with hydrogen donor substituents on the benzene ring, which has a strong capacity to scavenge free radicals (Fuentes et al., 2021; Xu et al., 2011). At present, the studies about the effects of QG are limited. Nevertheless, the effect of quercetin (3′,4′,5,6-pentahydroxy flavones) has been studied in depth. For instance, diets supplementation with quercetin linearly increased the BW, feed intake, and dry matter digestibility of broiler chickens from 1 to 35 days and improved growth performance (Abdel-Latif et al., 2021; Dang et al., 2022). Zhang and Kim (2020) reported that a quadratic response in BW gain was observed in broiler chickens, with a maximum in the 250 mg/kg quercetin group and no effect on nutrient digestibility. Yang et al. (2020) reported that diets supplementation with different dosages of quercetin (0%, 0.02%, 0.04%, or 0.06%) had no effect on growth performance. However, this effect was closely related to the type and dose of flavonoids, and the results reported were inconsistent. QG has one more phenolic hydroxyl than quercetin (3,3′,4′,5,6-pentahydroxy flavones) in its chemical structure, which has stronger biological activity and great potential for application as an additive (Xu et al., 2011). To date, there are few studies on the effects of QG on broiler chickens. In this study, we found that the addition of 100 mg/kg QG improved the BW at d 21 and the ADG and ADFI of broiler chickens in the starter period (1–21 days) increased quadratically. As well, the addition of QG improved the digestibility and absorption of nutrients, which may contribute to improving growth performance. The probable reason for growth promotion may be that flavones could increase the concentration of insulin-like growth factor-1 and promote protein synthesis in the muscle, which may contribute to the growth promotion of broilers (Kamboh & Zhu, 2013; Ouyang et al., 2016). No differences were observed in the growth performance in grower and overall periods. The various results on the effects of QG or quercetin might be due to the type of chicken, growth phases, diets, or different supplementation doses of additives. Diets supplementation with 200 mg/kg QG did not show positive effects, which may be due to high dosages of flavonoids acting as mutagens, pro-oxidants that produce free radicals, and inhibitors of key enzymes in hormone metabolism (Skibola & Smith, 2000). In this study, QG supplementation had a positive effect on early growth of broiler, while had no effect on grower phase and whole phase. This may be because the immune system of broilers in starter phase was not well developed and the anti-stress ability was weak (Proszkowiec-Weglarz et al., 2020). Further research is warranted to elucidate the underlying mechanisms of QG's effects and to evaluate its long-term benefits in various poultry production systems.
Feed costs in poultry production account for approximately 70% of total costs, and improving feed utilization to reduce costs is critical in farming (Banson et al., 2015). Quercetin enhanced protein absorption by increasing the internalization of oligopeptides in intestinal villi cells. This effect was associated with the flavonol backbone (Chen et al., 2021). Furthermore, flavonol compounds could upregulate the mRNA expression of nutrient transporter proteins such as glucose transporter 2 (GLUT2), fatty acid synthase (FAS), and peptide transporter 1 (PEPT1) (Abdel-Latif et al., 2021), which may be responsible for promoting the digestion and absorption of nutrients. This study found that diets supplementation with QG linearly or quadratically improved the digestibility of nutrients. However, whether QG improves digestibility by affecting the transporter carrier needs to be further investigated.
Physical indicators such as meat color, water holding capacity, shear force, and pH are commonly used to assess the quality of meat (Baéza et al., 2022). Wang et al. (2022) found that supplementation with 0.4 g/kg of quercetin improved the pH 45 min and L* value of the thigh muscle and reduced the shearing force of the thigh and breast muscles and drip loss of the thigh muscle. Goliomytis et al. (2014) found a linear effect of L* and a* (lower L*) on breast muscle with the addition of quercetin, which increased from 0 to 1 g/kg, which was consistent with our study. In this study, we found that the addition of 50 and 200 mg/kg QG decreased the L* value of breast muscle compared with 100 mg/kg QG group, and the addition of QG decreased the L* value of leg muscle compared with control group. The results of this study indicated that the effect of QG on meat quality only affected meat color. Meat color is the sensory attribute that most influences consumers' willingness to purchase, and consumption preference for meat color varies according to demographic factors and the consumption habits of different regions (Carvalho et al., 2021). The color of the meat surface is dominated by the pigment myoglobin, which changes color according to its biochemical state, especially the degree of oxidation or reduction of myoglobin (Purslow et al., 2020). It reduces myoglobin oxidation and improves meat color when muscles are kept away from free radical attack (Liu et al., 2021). GSH can directly react with hydroxyl (OH•) and O2− or donate electrons for the reduction of H2O2 (Chen et al., 2022). In this study, we found that the correlation analysis between antioxidants and meat quality showed a negative correlation between GSH-Px and L* value, and the pH 24 h was positively correlated with T-AOC content for leg muscles. Rupasinghe et al. (2010) reported that quercetin metabolites, such as quercetin glucosinolates and sulphonates, appeared to accumulate in breast and thigh muscles after 3-day diets and improved the antioxidant effects. QG supplementation may have the potential to regulate the antioxidant status of muscles and improve the color of meat. However, the improvement in meat quality is limited to meat color. The exact mechanism of action needs further study. The addition of QG had no effect on cooking loss, drip loss, or shear force. This difference may be caused by differences in the breeds, muscle composition, or age of broilers.
The immune organ index is always used to reflect the level of immune response in broiler chickens (Zhu et al., 2023). A decrease in immune organ weight represents immunosuppression, while a suitable increase in immune organ weight is an indication of immune enhancement (Hussain et al., 2012). Yang et al. (2020) found that the addition of 0.06% quercetin could increase the spleen index and thymus index. However, this study found that the addition of QG had no effect on the liver, spleen, or bursa of broiler chickens, and the addition of 25 mg/kg QG increased the thymus index. The thymus serves as an immune defense against foreign antigens and an immune tolerance to self-antigens (Farley et al., 2013). Quercetin acts primarily on dendritic cells in thymic stromal cells, targeting many intracellular signaling kinases and phosphatases, enzymes, and membrane proteins that are often essential for specific cellular functions, thereby regulating the immune system (Chirumbolo, 2010). Low dose of QG increased thymus index. However, whether it could enhance immunity and the exact mechanism remains unclear. The intestine is not only an important organ for the digestion and absorption of nutrients but also the largest immune organ (Zou et al., 2017). Maintaining the proper acidity required for normal physiological function of the digestive system is an important measure to ensure health, and when the pH value in the intestine is elevated, it causes rapid proliferation of E. coli bacteria, causing intestinal diseases (Xiong et al., 2019). Acidic environments are ideal for the growth and reproduction of beneficial flora, such as Lactobacillus. Lactic acid is produced by lactic acid bacteria, and a high concentration of lactic acid lowers the pH of the culture solution (Abdel-Latif et al., 2021; Hinton et al., 1992). In this study, compared with control group, diets QG supplementation did not affect the pH of intestinal digesta.
Flavonoids are well known for their antioxidant activity, including scavenging free radicals, hydroxyl radicals, and superoxide by single-electron transfer (Liao et al., 2018). Previous study found that the addition of quercetin to broiler diets increased the antioxidant capacity (Sun et al., 2020). QG has one more hydroxyl group than quercetin and shows strong antioxidant activity, which may be attributed to the hexahydroxy (-OH) group in its structure to reduce the formation and scavenge of free radicals (Bulut & Yilmaz, 2021). In addition, the oxygen atom on the 4-carbonyl group of the parent nucleus of QG shows a strong coordination ability, and its polyhydroxy spatial structure facilitates the formation of metal complexes of various structures (Xu et al., 2011), which is a vital source of the biological activities of QG, such as antioxidant activity. Antioxidant enzymes such as SOD, CAT, and GSH-Px mainly contribute to the enhancement of cellular antioxidant defense against oxidative stress (Yan et al., 2010). In this study, the addition of 50 and 100 mg/kg QG increased serum GSH-Px activity, and 50 mg/kg QG supplementation had higher serum CAT activity than 25 mg/kg QG group. GSH-Px and CAT, as free radical scavenging enzymes, play a vital role in scavenging lipid hydroperoxide and superoxide and alleviating the damage of organic hydrogen peroxide to the body (Liao et al., 2018). A previous study showed that QG exert antioxidant effects through the Keap1/Nrf2/ARE signal pathway in rabbits (Wu et al., 2023). While, the exact mechanism of antioxidant effect of QG in broilers needs further study. We found that QG had strong antioxidant abilities, which may contribute to improved growth performance, digestibility of nutrients, and meat quality.
In summary, this study demonstrated that diets supplementation with 50–100 mg/kg QG effectively improved the growth performance in the starter phase, improved the nutrient digestibility, antioxidant capacity and meat color in Cobb broilers. Therefore, as a new antioxidant, QG may have excellent application prospects in production of broiler chickens.
ACKNOWLEDGMENTS
This study was supported by the Hebei Natural Science Foundation (Grant No. C2022204218), National Natural Science Foundation of China (Grant No. 32302787), and the Earmarked Fund for Hebei Poultry Innovation Team of Modern Agroindustry Technology Research (Grant No. HBCT2023210408).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




