PMEL p.L18del associates with beef quality of Kumamoto sub-breed of Japanese Brown cattle
Abstract
Japanese Brown cattle is the second most popular breed among Wagyu breeds and raised mainly in Kumamoto and Kochi Prefectures. Typical coat color of the Kumamoto sub-breed is solid brown, but individuals with diluted coat color are sometimes born. We previously detected four SNPs in PMEL gene and identified p.L18del as the causative polymorphism of this diluted phenotype. The current study examined the association between the SNPs in PMEL gene and carcass traits of the Kumamoto sub-breed. Our association analysis revealed that p.L18del had significant effects on BMS (p = 0.0263), meat brightness (p = 0.0179), meat firmness (p = 0.0102), and meat texture (p = 0.0252) and that del allele of this SNP might be useful to improve these carcass traits.
1 INTRODUCTION
Japanese Brown cattle is the second most popular breed among four Wagyu breeds, Japanese domestic beef cattle breeds, and raised mainly in Kumamoto and Kochi Prefectures. The Kumamoto sub-breed has unique characteristics including greater growth rate compared with Japanese Black cattle, the most popular Wagyu breed (Sasaki et al., 2006). In Japan, beef cattle are evaluated with combination of yield grade and quality grade, expected cutability and palatability (Polkinghorne & Thompson, 2010), and, therefore, one of the breeding objectives of the Kumamoto sub-breed is to improve carcass traits related to these grades (Sasaki et al., 2006; Sumio, 2007). For this purpose, we previously suggested that the single nucleotide polymorphisms (SNPs) in Leptin (LEP) gene might be useful as DNA markers to improve beef marbling standard (BMS) and other traits of this sub-breed (Matsumoto et al., 2022). Development of additional DNA markers will be required to fasten the breeding of the Kumamoto sub-breed.
Two SNPs in melanocortin 1 receptor (MC1R) gene make typical coat color of the Kumamoto sub-breed solid brown (Matsumoto et al., 2020), but individuals with diluted coat color are sometimes born. We previously identified pre-melanosome protein (PMEL) as the causative gene of this diluted phenotype (Kimura et al., 2022). PMEL gene is mainly expressed in melanocyte, and its translated product makes melanosome within melanocyte as a pigment synthesis organelle (Watt et al., 2013). Therefore, SNPs in PMEL gene have been known to affect coat color of various animal species, including mouse, dog, and horse (Andersson et al., 2013; Kwon et al., 1994; Langevin et al., 2018).
Bovine PMEL gene is composed of 12 exons spanning 8813 bp on Bos taurus autosome (BTA) 5. Our previous study suggested that four SNPs (p.L18del: rs385468954, p.G22A: rs718553050, p.S36L: rs380609136, and p.A612E: rs378894329) might be polymorphic in the Kumamoto sub-breed (Kimura et al., 2022). Among these SNPs, p.L18del and p.G22A have been reported to cause coat color dilution. Animals with del/del type of p.L18del show diluted coat color in Highland cattle, as well as the Kumamoto sub-breed (Schmutz & Dreger, 2013). Although p.G22A have been reported to associate with coat color dilution of Charolais cattle (Gutiérrez-Gil et al., 2007), this SNP is unlikely to be involved in the diluted phenotype of the Kumamoto sub-breed (Kimura et al., 2022). As far as we know, influence of the other two SNPs on the gene functions and phenotype has not been reported.
Diluted cattle in the Kumamoto sub-breed are rumored to show better meat quality, although no one has tested this rumor scientifically. Furthermore, association between SNPs in PMEL gene and birth weight of Simmental × Holstein crossbred cattle has been reported (Wang et al., 2023). These findings suggest that PMEL gene might control not only coat color but also carcass traits of the Kumamoto sub-breed. The current study examined the association between the SNPs in PMEL gene and carcass traits of the Kumamoto sub-breed of Japanese Brown cattle.
2 MATERIALS AND METHODS
2.1 Animals
Genotypes of 313 Japanese brown cattle (212 steers and 101 heifers) were analyzed in this study. The average ages in months ± SD of this group at slaughter was 25.90 ± 1.28. Their adipose tissues around their kidneys were collected in 2018 and 2019 in the slaughterhouse (Kumamoto Chikusan Ryutsu Center Co. Ltd.), and their genomic DNA samples were extracted by standard phenol chloroform extraction. The approval of Animal Welfare and Ethics Board is not required, because all the samples analyzed in this study were obtained from carcasses.
Dressed carcass weight (kg), rib-eye area (cm2), rib thickness (cm), and subcutaneous fat thickness (cm) were measured for yield grade by official graders of the Japan Meat Grading Association. BMS (No. 1–12), beef color standards (BCSs) (No. 1–7), meat brightness (No. 1–7), meat firmness (No. 1–5), meat texture (No. 1–5), beef fat standards (BFSs) (No. 1–7), and fat luster and texture (No. 1–7) were evaluated for quality grade. Basic statistics of the animals analyzed in this study were previously reported (Matsumoto et al., 2022).
2.2 Genotyping
Genotyping of all concerned SNPs was conducted by the PCR-RFLP method reported by Kimura et al. (2022) (Figure 1). The PCR was performed with Go-Taq (Promega Corporation, Madison, WI, USA). The condition to amplify the region including p.L18del was 35 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. For the other SNPs, annealing temperature was changed to 60°C. The primer sequencings for p.L18del, p.G22A, p.S36L, and p.A612G were 5′-GGAAGGAAGAACAGGATGGATCT-3′ and 5′-TAGGGAGAGAAAAACCAGAGCAG-3′, 5′-ACTGTCAATGAGTAGCAGGATGTC-3′ and 5′-TGCACCCAAATCTTCATGTG-3′, 5′-AGGGAGTGAGTAATGATTTGAGGG-3′ and 5′-TCCTTCCCTATCTTCCTAAGTACA-3′, and 5′-AGGGAGCCAGGATCAAGACCAAG-3′ and 5′-AAGAGCACTCAGACCTGCTGTCC-3′, respectively. Obtained PCR products were digested by MboII (p.L18del), SfcI (p.G22A), BpuEI (p.S36L), and BspHI (p.A612G). These restriction enzymes were commercially purchased from New England Biolabs (Ipswich, MA, USA). After genotyping, HAPLOVIEW 4.0 software (https://www.broadinstitute.org/haploview/haploview) was applied to analyze linkage disequilibrium.
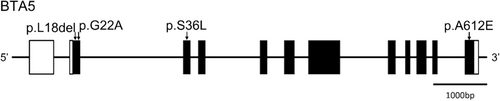
2.3 Statistical analysis
Stepwise selection was conducted to elucidate the factors contributing to all traits as previously reported (Matsumoto et al., 2022). In the model of each trait, SNPs, sex, age (in month: 25.90 ± 1.28 as mean ± SD), slaughtered year (two classes as 2018 and 2019), month (April to July, October, and December), pedigree (first to third sire: 260 classes), and farmer (39 classes) were added as explanatory variables. Stepwise selection was performed using the step-down method, based on the least Akaike information criterion (AIC). After the model selection, one-way analysis of variance (ANOVA) with least squares was performed to identify major factors. However, the animals with del/del allele of p.L18del were excluded from this analysis due to the limited number (n = 5). Differences between least square means of quality grades for genotypes were examined using t test. All the statistical analysis was performed using JMP14 (SAS Institute Inc, Cary, NC, USA).
3 RESULTS
Our genotyping using 313 cattle revealed that the frequencies of L/L type, L/del type, and del/del type of p.L18del were 0.68 (n = 214), 0.30 (n = 94), and 0.02 (n = 5), respectively. The minor allele of p.L18del was del allele with the frequency of 0.17. Because of their limited number, the individuals with del/del type were excluded from the association analysis. Besides, we applied only 100 animals in genotyping p.G22A, because all of 100 cattle were G/G homozygotes and it was suggested that this SNP was fixed in this sampling population. Therefore, p.G22A was excluded from the subsequent analysis. The frequencies of S/S type, S/L type, and L/L type of p.S36L were 0.17 (n = 54), 0.37 (n = 116), and 0.46 (n = 143), respectively, and those of A/A type, A/E type, and E/E type of p.A612E were 0.14 (n = 45), 0.45 (n = 140), and 0.41 (n = 128), respectively. The minor alleles of p.S36L and p.A612E were S allele with the frequency of 0.36. and A allele with the frequency of 0.37, respectively. Linkage disequilibrium (r2) between p.L18del and p.S36L was 0.06, that between p.S36L and p.A612E was 0.23, and that between p.L18del and p.A612E was 0.02, suggesting these SNPs were not in strong linkage disequilibrium.
After the model selection, pedigree and farmer were excepted from all models of each trait. F values of the factors selected in model of each trait were shown in Tables 1, S1, and S2. Only p.L18del showed effects on the traits related with quality grade. This SNP had significant effects on BMS (p = 0.0263), meat brightness (p = 0.0179), meat firmness (p = 0.0102), and meat texture (p = 0.0252). The t test revealed the detailed effects of p.L18del (Table 2). BMS of the animals with L/del type (4.80 ± 0.17) was higher than that of the animals with L/L type (4.35 ± 0.11). Similarly, meat brightness, meat firmness, and meat texture of the L/del animals (3.63 ± 0.08, 3.36 ± 0.09, and 3.76 ± 0.08, respectively) were significantly higher than those of the L/L animals (3.40 ± 0.05, 3.09 ± 0.06, and 3.55 ± 0.05, respectively). Although the association analysis was not performed due to their limited number (n = 5), BMS, meat brightness, meat firmness, and meat texture of del/del type were 5.20 ± 0.86, 4.00 ± 0.44, 3.60 ± 0.50, and 4.20 ± 0.37, respectively, suggesting the additive effect of p.L18del.
| Trait | p.L18del | Sex | Age | Year | Month |
|---|---|---|---|---|---|
| Yield grades | |||||
| Carcass weight | 0.43 | 99.74*** | 0.26 | 250.81 | 2.20 |
| Rib-eye area | 0.37 | 9.60** | 0.36 | 0.09 | 1.99 |
| Rib thickness | 1.44 | 3.94* | 0.71 | 4.42* | 0.08 |
| Subcutaneous fat thickness | 0.00 | 11.00** | 0.49 | 0.23 | 3.06 |
| Yield estimate | 1.01 | 0.31 | 2.86 | 0.11 | 0.007 |
| Quality grades | |||||
| BMS | 5.43* | 3.53 | 0.002 | 6.12* | 0.82 |
| BCS | 1.09 | 6.19* | 0.60 | 1.79 | 0.07 |
| Meat brightness | 5.61* | 4.30* | 0.40 | 0.70 | 3.16 |
| Meat firmness | 6.60* | 2.86 | 0.11 | 0.54 | 3.90* |
| Meat texture | 5.33* | 5.18* | 0.15 | 2.45 | 0.46 |
| Beef fat standard | 0.10 | 13.10*** | 1.07 | 5.46* | 4.99* |
| Fat luster and texture | 0.00 | 0.47 | 0.44 | 0.27 | 1.52 |
- Note: The number of degrees of freedom (df) was 2 in p.L18del, 1 in sex, 1 in age, 1 in year, and 5 month, respectively.
- * p < 0.05,
- ** p < 0.01, and
- *** p < 0.001.
| Trait | p.L18del | |
|---|---|---|
| L/L (n = 214) | L/del (n = 94) | |
| BMS | 4.35 ± 0.11b | 4.80 ± 0.17ª |
| Meat brightness | 3.40 ± 0.05b | 3.63 ± 0.08ª |
| Meat firmness | 3.09 ± 0.06b | 3.36 ± 0.09ª |
| Meat texture | 3.55 ± 0.05b | 3.76 ± 0.08ª |
- Note: Values are expressed as means with standard error by least squares estimates. Means with different superscripts (a and b) differ significantly at p < 0.05 (t test).
4 DISCUSSION
Our association analysis indicated that PMEL p.L18del might influence on BMS, meat brightness, meat firmness, and meat texture of the Kumamoto sub-breed and that del allele of this SNP might be useful to improve these carcass traits. Previous studies revealed that this allele dilutes coat color of several cattle breeds (Gutiérrez-Gil et al., 2007; Kimura et al., 2022; Liable et al., 2021; Schmutz & Dreger, 2013), suggesting a link between coat color and other phenotypes. In fact, influences of coat color on other phenotypes, such as temper, have been reported (Jacobs et al., 2016; Pérez-Guisado et al., 2006; Yamamuro & Shiraishi, 2011). For example, coat color of cocker spaniel is associated with dominant-aggressive behavior: Dogs with golden coat show more dominant behavior than those with other colors (Pérez-Guisado et al., 2006).
One of the genes determining coat color of Labrador retrievers is tyrosinase related protein 1 (TYRP1) (Schmutz et al., 2002). Coat color of Labrador retrievers homozygous for the recessive, brown allele of TYRP1 is chocolate, and the chocolate dogs are less trainable (van Rooy & Wade, 2019). These chocolate dogs have also been reported to show heavier weight and higher appetite than dogs with other coat colors (Wallis et al., 2023). Because TYRP1 is co-localized with PMEL in melanosome and these molecules interact with each other (Gao et al., 2022; Sturm et al., 2001), polymorphisms in PMEL gene might affect the function and/or localization of TYRP1, and PMEL p.L18del might change behaviors of the Kumamoto sub-breed, as well as their coat color. Relationships between behavior and meat quality have been reported in pig and chicken (Almasi et al., 2015; Ros-Freixedes et al., 2014). Behaviors of beef cattle also affect growth efficiency, and rough cattle show decreased carcass weight and meat quality (Café et al., 2011; Nkrumah et al., 2007), suggesting that the effects of PMEL p.L18del on carcass traits might be derived from behavioral changes.
PMEL p.R617C dilutes coat color of horse, and silver horses with C/C type of this SNP are suffered from multiple congenital ocular anomalies (MCOA) syndrome, a congenital eye disorder (Andersson et al., 2013). This SNP also causes behavioral change; silver horses are more cautious in novel situations probably because of visual defect (Brunberg et al., 2013). Furthermore, older horses with C allele are more likely to be myopic than the horses of the same age without this allele (Johansson et al., 2017). Dog is another example showing that PMEL governs coat color and behavior. Merle pattern is a random pattern in coat color produced by a 235 bp SINE insertion in PMEL gene. Dogs with the merle allele show high prevalence of deafness, resulting in their behavioral changes (Strain, 2015). Although we have not noticed any abnormality, diluted individuals of the Kumamoto sub-breed might possess impaired sense(s) of sight and/or hearing. Phenotypic analysis toward diluted cattle will be required in the future.
Coat color-related genes may interact with carcass traits-related genes. Agouti signaling protein (ASIP) is one of the well-studied coat color-related genes, and suppression of MC1R by ASIP represses eumelanin synthesis (Wolf Horrell et al., 2016). On the other hand, ASIP is one of the causative genes of obesity, and expression of adipogenic genes, including LEP which controls appetite and eating behavior, is regulated by ASIP (Kempf et al., 2022; Mynatt & Stephens, 2001). Because PMEL is a downstream gene of ASIP (Hida et al., 2020), PMEL is likely to participate in the regulation of LEP and other adipogenic genes. The SNPs in LEP affects carcass traits such as BMS, meat brightness, meat firmness, and BFS of the Kumamoto sub-breed (Matsumoto et al., 2022), suggesting that beef quality is influenced by LEP expression and/or function and that the effects of PMEL p.L18del in this study might be related with LEP and its related genes.
Beef cattle breeding aims to improve carcass yield and meat quality. In this respect, coat color dilution by PMEL p.L18del can be a useful and easy-to-use marker for the Kumamoto sub-breed of Japanese Brown cattle. However, coat color is important for registration of livestock, and, therefore, diluted individuals of the Kumamoto sub-breed are not allowed as breeding stocks. Our results suggested that genetic characteristics of the diluted individuals of the Kumamoto sub-breed should be elucidated and that the diluted cattle should be preserved based on this understanding.
ACKNOWLEDGMENTS
We appreciate the kind advice provided by Dr Kazuhiko Imakawa at the Research Institute of Agriculture at Tokai University. Part of this study was conducted with financial support from the Research Institute of Agriculture, Tokai University.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




