Analysis of the relationship between energy balance and properties of rumen fermentation of primiparous dairy cows during the perinatal period
Abstract
Short-chain fatty acids (SCFAs) produced in the rumen are key factors affecting dairy cows' energy balance (EB). This study aimed to quantitatively evaluate the effects of SCFAs production on EB in dairy cows. Primiparous dairy cows were divided into high non-esterified fatty acid (NEFA; group H) and low NEFA (group L) groups based on their blood NEFA levels at week 3 postpartum, which served as an indicator of EB. The amounts of SCFAs produced in the rumen, including acetate, propionate, and butyrate (SCFAsP), were calculated using the predicted rumen volume. Because there were no differences between the groups in SCFAsP/dry matter intake, whereas 4% fat-corrected milk (FCM)/SCFAsP was significantly higher in group H, it was suggested that more body fat was mobilized for milk production in group H. However, group L, which showed better EB, had propionate dominant and lower FCM/SCFAsP and milk energy/SCFAs energy at 3 and 7 weeks postpartum, indicating that group L had a better energy supply for milk production. These results suggest that SCFAsP produced by rumen fermentation and the composition of SCFAs in the rumen affect milk production and EB.
1 INTRODUCTION
The energy balance (EB) of dairy cows has been used as an indicator of energy excess or deficiency, expressed as the energy intake minus the energy requirement. Understanding rumen fermentation is important because cows use short-chain fatty acids (SCFAs) produced as energy sources by the anaerobic digestion of feed by various anaerobic microorganisms living in the rumen. Although rumen SCFAs concentrations are often used to characterize the general properties of rumen fermentation activity status and patterns (Seymour et al., 2005), Sutton et al. (2003) reported that SCFAs' concentrations in the rumen do not necessarily reflect SCFAs production. Hall (2013) also indicated that other approaches are needed to improve evaluation and interpretation for rumen fermentation (Hall, 2013), and there were many factors (e.g., absorption rate of SCFAs, passage rate of SCFAs, rumen fluid volume, etc.) affecting SCFAs concentration (Hall et al., 2015). Therefore, we attempted to solve this problem by converting SCFAs concentrations to quantifiable values.
A noteworthy characteristic of ruminants is that they use SCFAs by rumen fermentation as an energy source to produce milk and meat (Kruger Ben Shabat et al., 2016). Thus, the EB should consider rumen fermentation conditions to improve feed management. Energy intake is usually determined from feed intake, and energy requirements are calculated from body weight (BW), DG, and milk yield (NARO, 2017). In many cases, SCFAs produced in the rumen are treated as SCFAs concentration data; however, they are not used as quantitative data in the EB. This study aimed to quantify the effects of SCFAs production on EB in dairy cows by linking factors related to feed intake, rumen fermentation, and milk yield.
2 MATERIALS AND METHODS
2.1 Data
No actual experimental research on animals nor sampling were performed in this study. The data in this study were a reanalysis of the data obtained from experiments previously reported by Hayashi et al. (2014), in which animal studies were conducted according to the basic guidelines for animal experiments of the Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF), and are briefly described below.
The experimental studies were carried out in seven prefectural research institutes (Miyagi, Fukushima, Ibaraki, Saitama, Shizuoka, Gifu, and Kumamoto Prefectures) using primiparous Holstein dairy cows. The study was conducted twice, and 52 cows were tested from 6 weeks before parturition to 16 weeks postpartum. Cows were fed a total mixed ration (TMR) containing timothy hay, alfalfa hay cubes, and concentrate. During the first half of the prepartum, these cows were offered a diet consisting of 55% timothy hay, 25% alfalfa hay cubes, and 20% concentrate. During late prepartum, these cows were offered a diet consisting of 40% timothy hay, 25% hay cubes, and 35% concentrate. In both periods, forages were fed to meet 100% of the maintenance, 0.3 kg of daily gain, and total digestible nutrient (TDN) and crude protein (CP) requirements for pregnancy according to the Japanese Feeding Standard for Dairy Cattle (NARO, 2006). Post-parturition diets consisted of 24% timothy hay, 16% alfalfa hay cubes, and 60% concentrate and were fed ad libitum. The chemical compositions of the TMR fed post-parturition were 87.9 ± 0.19% (mean ± standard error) dry matter (DM), 16.1 ± 0.20% CP in DM, 3.5 ± 0.15% ether extract in DM, and 74.3 ± 0.27% TDN in DM.
Nutrient intake was calculated from the feed component values and the digestibility and non-digestibility rate in the Standard Tables of Feed Composition in Japan (Japan Livestock Industry Association, NARO, 2009). Milk yield was measured twice daily, and milk quality tests (fat, nonfat solids, and protein) were conducted weekly. Blood samples were collected from the jugular vein using heparinized tubes 1 week before, on the day of parturition, and at 13:00 at −1, 3, 7, and 16 weeks. The collected blood samples were immediately cooled and centrifuged (1500 × g, 10 min, 5°C), and plasma samples were stored below −30°C until general components analysis (plasma glucose, blood urea nitrogen, calcium, inorganic phosphorus, total cholesterol, total protein, albumin, aspartate aminotransferase, gamma-glutamyl transpeptidase, and non-esterified fatty acid; NEFA). Rumen fluid was collected at the same time as blood sampling. Collection was conducted via the mouth at 13:00 h. After pH measurement, the collected rumen fluid was filtered through a double layer of sterile gauze. Filtered rumen fluid was centrifuged (1500 × g, 15 min, 5°C) as samples for ammonia-form nitrogen (NH3-N) and SCFAs measurements and the residual samples were then stored at −20°C until analysis. The NH3-N was measured using the indophenol method. SCFAs were measured using gas chromatography (GC-14B; Shimadzu, Tokyo, Japan). The testing condition was as follows: packed column E-5476 (2.1 m long, 3.2 mm inner diameter; Shinwa-kako, Saitama, Japan), 250°C flame ionization detector, 250°C injector, and 115°C column temperatures. Helium and crotonic acid were used as the carrier gas and the inner standard, respectively.
2.2 Calculation for ruminal volume
The energy of each SCFA was calculated based on its molecular weight and caloric content. The molecular weight and calories of Ac were 60.05 g/mol and 3.5 kcal/g, respectively, those of Pr were 74.08 g/mol and 5 kcal/g, respectively, and those of Bu were 88.11 g/mol and 6 kcal/g, respectively (Akoh, 2017). The energy of fat-corrected milk (FCM) was calculated to be 750 kcal/kg (McDonald et al., 2002).
2.3 Statistical analysis
3 RESULTS
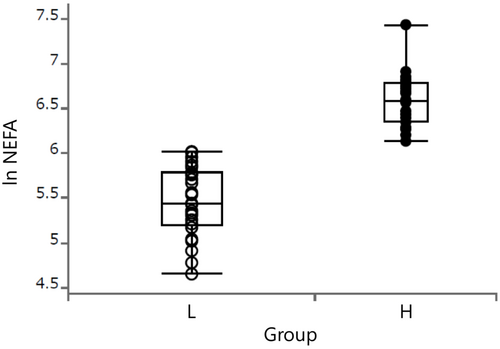
The parameters related to DMI, BW, milk, blood composition, and rumen fermentation are shown in Table 1. There were no significant differences between groups in DMI (P = 0.13) or milk production (P = 0.62). Dry matter intake/BW0.75 tended to interact with group and week (P = 0.051). After parturition, the DMI/BW0.75 of group H was lower than that of group L but gradually recovered to the same level as that of group L. BW had a significant interaction with group and week (P < 0.01). The postpartum BW recovery in group H was lower than that in group L (P < 0.01; BW 1 week before parturition was 100). Milk production showed no significant differences, but FCM tended to be higher in group H (P < 0.1). The sufficiency rate of TDN of group H, calculated using the Japanese feed standard, was lower than that of group L at weeks 3 and 7 (P < 0.05 and P < 0.1, respectively). NEFA was significantly different for blood parameters between the groups (P < 0.01), and all concentrations in group H were significantly higher than those in group L. Glucose concentrations were not significantly different between the groups (P = 0.2). The rumen SCFAs concentrations in group H tended to be lower than those in group L at weeks −1, 3, and 7 (P < 0.1). Ac (mM) showed no significant difference between the groups. The Pr (mM) and Bu (mM) levels in group H were lower than those in group L (P < 0.05). Consistent with these results, group H's Ac/Pr ratio tended to be higher than group L's ratio (P < 0.1). Rumen pH did not differ between these groups. No difference in SCFAsP (mol/d) was observed between the two groups (Table 1).
| Parameter | Weeks postpartum | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1 w | +3 w | +7 w | +16 w | P value | ||||||||||||
| LSM | ±SE | P | LSM | ±SE | P | LSM | ±SE | P | LSM | ±SE | P | Group | Week | G × W | ||
| Physical | ||||||||||||||||
| DMI (kg) | L | 10.3 | 0.38 | n.s. | 17.0 | 0.38 | n.s. | 19.7 | 0.38 | n.s. | 21.2 | 0.38 | n.s. | 0.13 | <0.01 | 0.18 |
| H | 10.2 | 0.45 | 15.6 | 0.44 | 18.9 | 0.44 | 20.8 | 0.46 | ||||||||
| DMI/BW0.75 (g) | L | 83 | 3.2 | n.s. | 155 | 3.2 | ** | 179 | 3.2 | n.s. | 183 | 3.2 | n.s. | 0.08 | <0.01 | 0.05 |
| H | 79 | 3.8 | 140 | 3.8 | 171 | 3.8 | 183 | 3.9 | ||||||||
| BW (kg) | L | 619 | 10.2 | * | 528 | 10.2 | n.s. | 533 | 10.2 | n.s. | 567 | 10.2 | n.s. | 0.57 | <0.01 | <0.01 |
| H | 653 | 11.9 | 537 | 11.9 | 533 | 11.9 | 557 | 11.9 | ||||||||
| BW change (%) | L | 100 | 0.0 | n.s. | 85 | 0.8 | * | 86 | 0.8 | ** | 92 | 0.8 | ** | <0.01 | <0.01 | <0.01 |
| H | 100 | 0.0 | 82 | 1.0 | 82 | 1.0 | 86 | 1.0 | ||||||||
| Milk production (kg) | L | - | - | - | 29.1 | 0.9 | n.s. | 32.0 | 0.9 | n.s. | 31.7 | 0.9 | n.s. | 0.62 | <0.01 | 0.40 |
| H | - | - | 30.0 | 1.1 | 33.2 | 1.1 | 31.5 | 1.1 | ||||||||
| FCM (kg/day) | L | - | - | - | 30.2 | 1.1 | * | 31.4 | 1.1 | n.s. | 29.4 | 1.1 | n.s. | 0.08 | 0.02 | 0.38 |
| H | - | - | 33.9 | 1.2 | 33.3 | 1.2 | 31.2 | 1.3 | ||||||||
| Milk fat (%) | L | - | - | - | 4.3 | 0.15 | ** | 3.9 | 0.15 | n.s. | 3.5 | 0.15 | † | 0.02 | <0.01 | 0.19 |
| H | - | - | 4.9 | 0.17 | 4.0 | 0.17 | 4.0 | 0.18 | ||||||||
| Milk protein (%) | L | - | - | - | 3.1 | 0.04 | n.s. | 3.0 | 0.04 | n.s. | 3.1 | 0.04 | n.s. | 0.65 | <0.01 | 0.18 |
| H | - | - | 3.2 | 0.05 | 3.0 | 0.05 | 3.1 | 0.05 | ||||||||
| SNF (%) | L | - | - | - | 8.6 | 0.06 | n.s. | 8.6 | 0.06 | n.s. | 8.7 | 0.06 | n.s. | 0.82 | <0.01 | 0.65 |
| H | - | - | 8.6 | 0.07 | 8.5 | 0.07 | 8.6 | 0.07 | ||||||||
| Sufficiency rate of TDN (%) | L | 101.8 | 2.3 | n.s. | 83.0 | 2.3 | ** | 92.7 | 2.3 | * | 101.5 | 2.3 | n.s. | 0.02 | <0.01 | 0.10 |
| H | 98.0 | 2.7 | 71.3 | 2.7 | 85.5 | 2.7 | 96.7 | 2.8 | ||||||||
| Blood | ||||||||||||||||
| Total protein (g/dL) | L | 6.0 | 0.12 | n.s. | 7.3 | 0.12 | n.s. | 7.4 | 0.12 | n.s. | 7.4 | 0.12 | n.s. | 0.48 | <0.01 | 0.81 |
| H | 5.9 | 0.14 | 7.2 | 0.14 | 7.3 | 0.14 | 7.4 | 0.14 | ||||||||
| Albumin (g/dL) | L | 3.5 | 0.06 | n.s. | 3.6 | 0.05 | n.s. | 3.7 | 0.05 | n.s. | 3.8 | 0.05 | n.s. | 0.24 | <0.01 | 0.53 |
| H | 3.5 | 0.06 | 3.8 | 0.06 | 3.8 | 0.06 | 3.8 | 0.06 | ||||||||
| BUN (g/dL) | L | 13.2 | 0.63 | n.s. | 11.9 | 0.62 | n.s. | 15.0 | 0.62 | n.s. | 15.4 | 0.62 | n.s. | 0.73 | <0.01 | 0.10 |
| H | 13.8 | 0.73 | 12.4 | 0.73 | 13.8 | 0.73 | 14.4 | 0.73 | ||||||||
| NEFA (μEq/L) | L | 230.3 | 32.23 | * | 250.9 | 31.77 | ** | 176.7 | 32.23 | ** | 123.0 | 31.77 | ** | <0.01 | <0.01 | <0.01 |
| H | 333.6 | 37.10 | 763.9 | 37.10 | 358.1 | 37.10 | 265.3 | 37.10 | ||||||||
| Glucose (mg/dL) | L | 72.0 | 1.40 | n.s. | 64.0 | 1.38 | n.s. | 67.3 | 1.38 | n.s. | 67.5 | 1.38 | n.s. | 0.20 | <0.01 | 0.38 |
| H | 71.8 | 1.61 | 59.8 | 1.61 | 64.9 | 1.61 | 66.0 | 1.61 | ||||||||
| T-Cho (mg/dL) | L | 70.6 | 8.07 | n.s. | 142.3 | 7.95 | n.s. | 209.7 | 7.95 | * | 257.9 | 7.95 | n.s. | 0.06 | <0.01 | 0.50 |
| H | 82.8 | 9.29 | 155.3 | 9.29 | 239.4 | 9.29 | 267.0 | 9.29 | ||||||||
| AST (U/L) | L | 44.8 | 5.42 | n.s. | 64.4 | 5.34 | n.s. | 68.0 | 5.34 | n.s. | 86.8 | 5.34 | n.s. | 0.40 | <0.01 | 0.37 |
| H | 46.5 | 6.24 | 79.1 | 6.24 | 72.5 | 6.24 | 84.6 | 6.24 | ||||||||
| GGT (U/L) | L | 25.5 | 2.25 | n.s. | 29.8 | 2.21 | n.s. | 31.2 | 2.21 | n.s. | 36.6 | 2.21 | n.s. | 0.39 | <0.01 | 0.69 |
| H | 25.5 | 2.59 | 34.4 | 2.59 | 33.6 | 2.59 | 37.6 | 2.59 | ||||||||
| Ca (mg/dL) | L | 11.1 | 0.28 | n.s. | 11.3 | 0.27 | n.s. | 11.7 | 0.27 | n.s. | 11.8 | 0.27 | n.s. | 0.89 | <0.01 | 0.30 |
| H | 11.2 | 0.32 | 11.4 | 0.32 | 12.0 | 0.32 | 11.6 | 0.32 | ||||||||
| iP (mg/dL) | L | 5.5 | 0.22 | n.s. | 5.4 | 0.22 | n.s. | 5.4 | 0.22 | n.s. | 5.8 | 0.22 | n.s. | 0.25 | 0.07 | 0.78 |
| H | 5.9 | 0.26 | 5.5 | 0.26 | 5.8 | 0.26 | 6.1 | 0.26 | ||||||||
| Rumen | ||||||||||||||||
| SCFAs (mM) | L | 83.8 | 4.07 | † | 84.6 | 3.97 | † | 87.4 | 3.97 | † | 87.9 | 3.97 | n.s. | 0.06 | 0.03 | 0.86 |
| H | 71.7 | 4.71 | 73.4 | 4.67 | 76.9 | 4.64 | 79.5 | 4.64 | ||||||||
| Ac (mM) | L | 56.6 | 2.54 | n.s. | 53.9 | 2.47 | n.s. | 56.2 | 2.49 | n.s. | 56.7 | 2.47 | n.s. | 0.13 | 0.17 | 0.89 |
| H | 50.1 | 2.94 | 48.9 | 2.91 | 50.9 | 2.89 | 52.5 | 2.89 | ||||||||
| Pr (mM) | L | 17.2 | 1.17 | * | 19.7 | 1.14 | * | 21.2 | 1.15 | * | 21.0 | 1.14 | † | 0.02 | <0.01 | 0.80 |
| H | 13.5 | 1.36 | 15.5 | 1.35 | 17.0 | 1.34 | 17.8 | 1.34 | ||||||||
| Bu (mM) | L | 9.9 | 0.64 | † | 11.1 | 0.62 | * | 10.8 | 0.63 | † | 10.2 | 0.62 | n.s. | 0.05 | 0.04 | 0.58 |
| H | 8.0 | 0.74 | 9.0 | 0.73 | 9.0 | 0.73 | 9.1 | 0.73 | ||||||||
| Ac (%) | L | 68.4 | 0.69 | n.s. | 64.2 | 0.67 | * | 64.3 | 0.67 | † | 65.0 | 0.67 | n.s. | 0.04 | <0.01 | 0.58 |
| H | 70.1 | 0.80 | 66.5 | 0.79 | 66.3 | 0.78 | 66.0 | 0.78 | ||||||||
| Pr (%) | L | 20.0 | 0.63 | n.s. | 22.9 | 0.61 | † | 23.7 | 0.61 | † | 23.6 | 0.61 | n.s. | 0.08 | <0.01 | 0.89 |
| H | 18.8 | 0.73 | 21.3 | 0.72 | 22.1 | 0.71 | 22.5 | 0.71 | ||||||||
| Bu (%) | L | 11.5 | 0.33 | n.s. | 12.9 | 0.31 | n.s. | 12.0 | 0.31 | n.s. | 11.5 | 0.31 | n.s. | 0.29 | <0.01 | 0.59 |
| H | 11.1 | 0.37 | 12.2 | 0.37 | 11.7 | 0.36 | 11.5 | 0.36 | ||||||||
| Ac/Pr ratio | L | 3.5 | 0.11 | n.s. | 2.9 | 0.10 | † | 2.8 | 0.10 | n.s. | 2.8 | 0.10 | n.s. | 0.07 | <0.01 | 0.85 |
| H | 3.8 | 0.12 | 3.2 | 0.12 | 3.1 | 0.12 | 3.0 | 0.12 | ||||||||
| SCFAsP (mol/day) | L | 44.6 | 1.78 | n.s. | 72.3 | 1.70 | n.s. | 83.7 | 1.71 | n.s. | 89.6 | 1.70 | n.s. | 0.24 | <0.01 | 0.13 |
| H | 45.3 | 2.05 | 66.5 | 2.01 | 80.5 | 1.98 | 88.3 | 2.05 | ||||||||
| Rumen (pH) | L | 6.7 | 0.08 | n.s. | 6.5 | 0.07 | n.s. | 6.5 | 0.07 | n.s. | 6.5 | 0.07 | n.s. | 0.12 | <0.01 | 0.36 |
| H | 6.9 | 0.09 | 6.7 | 0.09 | 6.6 | 0.09 | 6.6 | 0.09 | ||||||||
| NH3-N (mg/dL) | L | 4.6 | 0.58 | n.s. | 5.5 | 0.55 | n.s. | 5.3 | 0.55 | n.s. | 5.9 | 0.55 | * | 0.04 | 0.31 | 0.75 |
| H | 3.6 | 0.66 | 4.1 | 0.64 | 4.0 | 0.64 | 4.0 | 0.64 | ||||||||
- Abbreviations: Ac, acetate; AST, aspartate aminotransferase; Bu, butyrate; BUN, blood urea nitrogen; BW, body weight; Ca, calcium; DMI, dry matter; FCM, 4% fat corrected milk; GGT, gamma-glutamyl transpeptidase; H, high NEFA group; iP, inorganic phosphorus; L, low NEFA group; NEFA, non-esterified fatty acids; NH3-N, ammonia nitrogen; Pr, propionate; SCFA, short chain fatty acid; SCFAsP, SCFAs production; SNF, solids not fat; T-cho, total cholesterol; TDNI/FS, total digestive nutrients intake/feed standard.
- † Significant difference: P < 0.1.
- * Significant difference: P < 0.05.
- ** Significant difference: P < 0.01.
Table 2 shows parameters relating to [H]. [HUS] production (mol/d) of group H was lower than that of group L at weeks 3 and 7 (P < 0.05), that of group H tended to be lower than that of group L at week 16 (P < 0.1). The HUM/HUS ratio of group H was higher than that of group L (P < 0.05). Estimated rumen CH4 concentration (mM) and CH4P (mol/d) showed no statistically significant differences between these groups (P = 0.19 and P = 0.96, Table 2). CH4/DMI (mol/kg) of group H tended to be higher than that of group L (P < 0.1). PRV and TTOR showed no significant difference between these groups (Table 2).
| −1 w | +3 w | +7 w | +16 w | P value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSM | ±SE | P | LSM | ±SE | P | LSM | ±SE | P | LSM | ±SE | P | Group | Week | G × W | ||
| HU (mM) | L | 153.0 | 7.50 | † | 154.5 | 7.32 | † | 159.0 | 7.36 | † | 157.7 | 7.32 | n.s. | 0.07 | 0.14 | 0.81 |
| H | 131.3 | 8.69 | 134.2 | 8.62 | 139.3 | 8.55 | 143.5 | 8.55 | ||||||||
| HUS (mM) | L | 54.3 | 3.44 | * | 61.5 | 3.36 | * | 64.0 | 3.38 | * | 62.4 | 3.36 | n.s. | 0.02 | <0.01 | 0.66 |
| H | 43.0 | 3.99 | 48.9 | 3.96 | 52.0 | 3.93 | 53.9 | 3.93 | ||||||||
| HUM (mM) | L | 98.7 | 4.52 | n.s. | 93.0 | 4.38 | n.s. | 95.0 | 4.41 | n.s. | 95.3 | 4.38 | n.s. | 0.19 | 0.4 | 0.87 |
| H | 88.3 | 5.22 | 85.4 | 5.17 | 87.3 | 5.12 | 89.6 | 5.12 | ||||||||
| HU (mol/day) | L | 81.5 | 3.41 | n.s. | 132.2 | 3.25 | n.s. | 150.9 | 3.28 | n.s. | 160.9 | 3.25 | n.s. | 0.31 | <0.01 | 0.14 |
| H | 83.0 | 3.92 | 124.4 | 3.85 | 146.1 | 3.79 | 159.3 | 3.92 | ||||||||
| HUS (mol/day) | L | 28.1 | 1.22 | n.s. | 51.4 | 1.15 | ** | 59.3 | 1.16 | ** | 62.3 | 1.15 | † | <0.01 | <0.01 | 0.08 |
| H | 27.0 | 1.40 | 44.7 | 1.37 | 54.2 | 1.34 | 58.9 | 1.40 | ||||||||
| HUM (mol/day) | L | 53.3 | 2.85 | n.s. | 80.8 | 2.73 | n.s. | 91.5 | 2.73 | n.s. | 98.5 | 2.73 | n.s. | 0.95 | <0.01 | 0.39 |
| H | 55.9 | 3.28 | 76.8 | 3.23 | 91.9 | 3.19 | 100.2 | 3.28 | ||||||||
| HUM/HUS ratio (mol/mol) | L | 1.95 | 0.06 | n.s. | 1.58 | 0.05 | * | 1.56 | 0.06 | n.s. | 1.59 | 0.05 | n.s. | 0.05 | <0.01 | 0.75 |
| H | 2.08 | 0.07 | 1.76 | 0.07 | 1.70 | 0.06 | 1.68 | 0.06 | ||||||||
| CH4 (mM) | L | 24.7 | 1.13 | n.s. | 23.2 | 1.10 | n.s. | 23.7 | 1.10 | n.s. | 23.8 | 1.10 | n.s. | 0.19 | 0.44 | 0.87 |
| H | 22.1 | 1.30 | 21.3 | 1.29 | 21.8 | 1.28 | 22.4 | 1.28 | ||||||||
| CH4P (mol/day) | L | 13.3 | 0.71 | n.s. | 20.2 | 0.68 | n.s. | 22.9 | 0.69 | n.s. | 24.6 | 0.68 | n.s. | 0.96 | <0.01 | 0.38 |
| H | 14.0 | 0.82 | 19.2 | 0.81 | 23.0 | 0.80 | 25.1 | 0.82 | ||||||||
| CH4P/DMI (mol/kg) | L | 1.32 | 0.02 | n.s. | 1.18 | 0.02 | † | 1.15 | 0.02 | n.s. | 1.15 | 0.02 | n.s. | 0.08 | <0.01 | 0.96 |
| H | 1.37 | 0.03 | 1.25 | 0.03 | 1.21 | 0.03 | 1.20 | 0.03 | ||||||||
| PRV (L/day) | L | 572 | 56.1 | n.s. | 957 | 54.5 | n.s. | 1,033 | 54.9 | n.s. | 1,106 | 66.9 | n.s. | 0.55 | <0.01 | 0.47 |
| H | 667 | 64.9 | 945 | 64.2 | 1,083 | 63.7 | 1,152 | 64.9 | ||||||||
| TTOR | L | 4.56 | 0.45 | n.s. | 8.60 | 0.44 | n.s. | 9.24 | 0.44 | n.s. | 9.45 | 0.44 | n.s. | 0.47 | <0.01 | 0.50 |
| H | 5.15 | 0.52 | 8.48 | 0.51 | 9.78 | 0.51 | 10.11 | 0.52 | ||||||||
- Abbreviations: CH4, methane concentration (mM); CH4P, methane production (mol/day); DMI, dry matter, PRV; predicted rumen volume; H, high NEFA group; HU, [H] utilized; HUM, [H] utilized in methane; HUS, [H] utilized in SCFAs; L, low NEFA group; LSM, least squares mean; P, P value; SE, standard error of the mean; TTOR, the turnover rates of the ruminal liquid fraction per unit MBW per day.
- † Significant difference: <0.1.
- * Significant difference: <0.05.
- ** Significant difference: <0.01.
The relationships between DMI, SCFAsP, and FCM are shown in Figure 2. FCM/DMI (kg/kg; Figure 2a) was significantly different between the groups (P < 0.01) and was significantly higher in group H at week 3 (P < 0.01). SCFAsP/DMI (mol/kg; Figure 2b) did not differ significantly between groups. The FCM/SCFAsP (kg/mol; Figure 2c) was significantly higher in group H (P < 0.01) than in group L, especially at week 3 (P < 0.01).
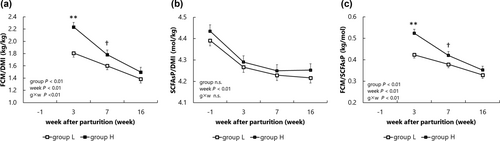
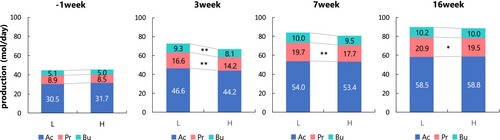
Figure 3 shows a comparison of SCFAs production between groups. The Pr (mol/d) of group H was lower at weeks 3 and 7 than that of group L, and week 16 did not cause statistical differences in SCFAsP. The Bu (mol/d) in group H was lower than that in group L at week 3 (P < 0.01).
Gross energy intake (GEI) and energy of SCFAs (SCFAsE), FCM (milkE), and CH4 (CH4E) are shown in Table 3. There were no differences in the GEI between these groups. Energy of SCFAs levels tended to differ between these groups (P < 0.1) and were higher in group L at weeks 3 (P < 0.05) and 7 (P < 0.1). Energy of milk tended to differ between these groups (P < 0.1) and was significantly lower in group L at week 3 (P < 0.05). MilkE/GEI (Mcal/Mcal, feed efficiency) in group H at weeks 3 and 7 were higher than in group L (P < 0.01 and P < 0.1, respectively). SCFAsE/GEI (Mcal/Mcal) was not different between these groups. MilkE/SCFAsE (Mcal/Mcal) was significantly different between these groups, week, and interaction (P < 0.01). No differences were observed in the amount of energy lost concerning CH4. CH4E/GEI (Mcal/Mcal) tended to differ between these groups and that of group L at week 3 was lower than that of group H (P < 0.1).
| −1 w | +3 w | +7 w | +16 w | P value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSM | ±SE | P | LSM | ±SE | P | LSM | ±SE | P | LSM | ±SE | P | Group | Week | G * W | ||
| GEI (Mcal/day) | L | 46.1 | 1.70 | n.s. | 76.2 | 1.70 | n.s. | 88.7 | 1.70 | n.s. | 95.4 | 1.70 | n.s. | 0.13 | <0.01 | 0.18 |
| H | 45.9 | 2.01 | 70.0 | 1.98 | 85.1 | 1.98 | 93.4 | 2.05 | ||||||||
| SCFAsE (Mcal/day) | L | 12.5 | 0.48 | n.s. | 20.8 | 0.45 | ** | 23.9 | 0.46 | † | 25.4 | 0.45 | n.s. | 0.08 | <0.01 | 0.09 |
| H | 12.5 | 0.55 | 18.8 | 0.54 | 22.8 | 0.53 | 24.9 | 0.55 | ||||||||
| MilkE (Mcal/day) | L | - | - | - | 22.6 | 0.79 | * | 23.6 | 0.79 | n.s. | 22.1 | 0.79 | n.s. | 0.08 | 0.02 | 0.38 |
| H | - | - | 25.4 | 0.92 | 25.0 | 0.92 | 23.4 | 0.95 | ||||||||
| MilkE/GEI | L | - | - | - | 0.302 | 0.0108 | ** | 0.267 | 0.0108 | † | 0.231 | 0.0108 | n.s. | <0.01 | <0.01 | <0.01 |
| H | - | - | 0.373 | 0.0126 | 0.297 | 0.0126 | 0.250 | 0.0130 | ||||||||
| SCFAsE/GEI | L | 0.272 | 0.0017 | n.s. | 0.273 | 0.0016 | n.s. | 0.269 | 0.0016 | n.s. | 0.267 | 0.0016 | n.s. | 0.43 | <0.01 | 0.79 |
| H | 0.272 | 0.0019 | 0.270 | 0.0019 | 0.267 | 0.0018 | 0.266 | 0.0019 | ||||||||
| milkE/SCFAsE | L | - | - | - | 1.103 | 0.0396 | ** | 0.992 | 0.0400 | * | 0.868 | 0.0396 | n.s. | <0.01 | <0.01 | <0.01 |
| H | - | - | 1.383 | 0.0470 | 1.116 | 0.0463 | 0.940 | 0.0478 | ||||||||
| CH4E (Mcal/day) | L | 2.83 | 0.151 | n.s. | 4.28 | 0.145 | n.s. | 4.85 | 0.146 | n.s. | 5.22 | 0.145 | n.s. | 0.95 | <0.01 | 0.39 |
| H | 2.97 | 0.174 | 4.07 | 0.171 | 4.87 | 0.169 | 5.31 | 0.174 | ||||||||
| CH4E/GEI | L | 0.062 | 0.0011 | n.s. | 0.056 | 0.0011 | † | 0.054 | 0.0011 | n.s. | 0.054 | 0.0011 | n.s. | 0.08 | <0.01 | 0.96 |
| H | 0.065 | 0.0013 | 0.059 | 0.0013 | 0.057 | 0.0013 | 0.057 | 0.0013 | ||||||||
- Abbreviation: CH4E, methane gas energy; DMI, dry matter intake; FCME, 4% fat corrected milk energy; GEI, gross energy intake; LSM, least squares mean; P, P value; SCFAsE, short chain fatty acids energy (Mcal); SE, standard error of the mean.
- † Significant difference: P < 0.1.
- * Significant difference: P < 0.05.
- ** Significant difference: P < 0.01.
4 DISCUSSION
The total amount of SCFAs in the rumen, which are the products of rumen fermentation, is a key factor in milk production. The estimation of SCFAsP using isotopes is currently the most accurate method; however, according to Sutton et al. (2003), the method requires complicated processing to isolate the substance of interest from biological samples. However, methane is not readily dissolved into the rumen liquid fraction (Speight, 2017) and is rarely absorbed through the rumen wall. Therefore, methane could be used as a stable indicator of ruminal fermentation (Mitsumori et al., 2019). This study estimated SCFAsP using methane to determine rumen volume, and its relationship with EB was further examined. Hristov et al. (2013) and Mitsumori et al. (2019) estimated CH4P using DMI as a variable. We used the estimation equation by Williams et al. (2019), which considers rumen fermentation patterns because the ratio of roughage to concentrate per DMI is expected to change owing to feed changes before and after parturition.
NEFA concentrations reflect the body fat released into the blood as long-chain fatty acids owing to energy deficiency. Therefore, NEFA concentrations are used as an indicator to determine short-term energy balance (van Knegsel et al., 2005). In general, the normal range of NEFA is 185 ± 77 μEq/L in the dry period, 225 ± 173 Eq/L in the early lactation period, and 135 ± 42 μEq/L or less in the mid-lactation period (Inokuma et al., 2017). This study showed that cows in group H showing negative energy balance (NEB) at week 3 continued to develop NEB until week 16. Primiparous cows distribute more energy for body growth than multiparous cows (Wathes, Cheng, et al., 2007); hence, they are less likely to develop NEB caused by milk production than multiparous cows are. Nevertheless, these are still primiparous cows categorized as NEB owing to high NEFA concentrations; therefore, this study examined the factors contributing to EB in primiparous cows. Fluctuations in glucose are more tightly controlled than those in NEFA (Wathes, Fenwick, et al., 2007) and decrease under severe low-energy conditions (van Knegsel et al., 2005). In this study, glucose decreased at week 3 regardless of group, but concentrations were within the normal range (group L: 64.0 mg/dL, group H: 59.8 mg/dL).
Lactating cows generally reach peak milk yield approximately 4–5 weeks after parturition, whereas DMI peaks approximately 8–10 weeks after. This discrepancy could be one reason for the NEB (NARO, 2017). During NEB, cows lose weight as body fat mobilization compensates for the energy required for milk production. Group H had higher NEFA levels and was 15% lower in BW at weeks 7 and 16 than at week −1. Body weight recovery in group H was slow, suggesting that NEB persisted for a long time. Group H produced more FCM despite having the same feed composition and low TDN sufficiency rates after parturition, suggesting that group H had a slower BW recovery and a longer NEB period than group L.
In general, changes in the ratio of roughage and concentrate in feed affect rumen fermentation (Janssen, 2010; Johnson & Johnson, 1995; Sutton et al., 2003). In this study, the composition of SCFAs differed among individuals despite having the same feed composition, indicating that rumen fermentation patterns varied among individuals, even without differences in feed composition. Furthermore, cows in group L, which tended to be propionate dominant, had milder NEB before and after parturition, even when fed the same feed.
The SCFAs concentration in the rumen is commonly measured to determine rumen fermentation parameters (Seymour et al., 2005). However, the SCFAs concentration in rumen fluid is not sufficient as an indicator of rumen microbial activity because of the difference between the rate of production by rumen microorganisms and the rates of absorption through the rumen wall and disappearance into the digestive tract (Dijkstra et al., 1993; Hall et al., 2015). In this study, SCFAsP was calculated from SCFAs concentrations and PRV. Results of SCFAsP ranged from 45.2 to 89.6 mol/d, which is close to the values reported by Sharp et al. (1982) and Dieho et al. (2016) (45 to 81.8 mol/d and 49 to 126 mol/d, respectively). Furthermore, the SCFAsP/DMI was calculated to be between 4.22 and 4.43 mol/kg. Previously reported SCFAsP/DMI ranged from 3.4 to 5.8 mol/kg (Whitelaw et al., 1970), and the values obtained in the present study fell within this range. Although the SCFAs concentrations in group L tended to be higher than those in group H, there were no significant differences in SCFAsP between these groups. Both groups produced the same amount of SCFAsP, suggesting that the SCFAs composition affects NEB.
Microorganisms rapidly remove [H] in the rumen. Methanogenesis is one of the main pathways of [H] removal. Acetate and butyrate production promotes methanogenesis because they release [H] during biosynthesis; however, propionate competes with CH4 because it utilizes [H] (Ungerfeld, 2020). There was no difference in HUM (mol/d) and CH4 (mol/d) between the two groups; however, HUS (mol/d) was higher in group L after parturition. This means that more [H] was used in the biosynthesis of propionate. Propionate production (mol/d) was significantly higher in group L after parturition, and the energy of SCFAs (Mcal/d, SCFAsE) was significantly higher in group L at 3 weeks postpartum and tended to be higher at 7 weeks. The better EB in group L may reflect the SCFAsE (Mcal/d) produced in the rumen.
The quantitative relationships between feed, rumen fermentation, and milk production were also analyzed using DMI, SCFAsP, and FCM. Regarding the relationship between DMI and FCM, the FCM was significantly higher in group H at week 3; however, no significant differences were observed at weeks 7 and 16. In contrast, the FCM/DMI was significantly higher in group H during the entire period. This indicated higher milk production per DMI, suggesting more body fat mobilization, leading to higher NEFA, that is, NEB status, in group H. Considering that there were no differences in SCFAsP/DMI (mol/kg) between the groups, the SCFAsP produced per DMI did not seem directly related to EB. Because FCM/SCFAsP and FCM/DMI were higher in group H at weeks 3 and 7, group H produced more milk relative to the amount of SCFAs produced in the rumen, which could be one of the reasons why more fat was mobilized for milk production. Hoedemaker et al. (2004) showed that feeding glucogenic nutrients decreased NEFA and BHBA concentrations in the blood. Increased utilization of glucogenic nutrients improves EB and reduces the risk of metabolic and reproductive disorders (van Knegsel et al., 2007). These reports indicated propionate, a glucogenic SCFA, is closely associated with energy sufficiency. The glucose required for milk production is mainly derived from feed intake and body fat mobilization. Although the proportion of glucose sources was unclear in this study, a significant difference in propionate production was observed between the groups. We also examined the relationship between feed, rumen fermentation, and milk production from an energy perspective. There were no significant differences in the SCFAsP levels between the groups. However, SCFAsE was higher in group L at weeks 3 and 7, presumably owing to significantly higher propionate and butyrate concentrations in group L, which may be one of the factors that had a positive effect on EB in group L. Interestingly, the ratio of SCFAs energy to feed energy (SCFAsE/GEI) was nearly the same for all observations, that is, 0.27 for any week, which approximated the value (0.315) obtained by Whitelaw et al. (1970).
In conclusion, the present study demonstrates that SCFAsP can be calculated using PRV. Because there were no differences between the groups in SCFAsP/DMI, and FCM/SCFAsP was significantly higher in group H, it is suggested that more body fat was mobilized for milk production in group H. However, the group with better EB (group L) had propionate dominant and showed lower FCM/SCFAsP, indicating a better energy supply for milk production. Further research is required to clarify the detailed mechanisms underlying EB and rumen fermentation.
ACKNOWLEDGMENTS
We express our deepest gratitude to the researchers at the prefectural research institutes (Miyagi, Fukushima, Ibaraki, Saitama, Shizuoka, Gifu, and Kumamoto Prefectures) for collecting the data used in this study. We thank Mr. Ishibashi, Stat Labo Co., Ltd., for their assistance with the statistical analysis. This study was supported by the Cabinet Office, Government of Japan, Moonshot Research and Development Program for Agriculture, Forestry and Fisheries (funding agency: Bio-oriented Technology Research Advancement Institution) (No. JPJ009237). The data used in this study were provided from studies conducted by Hayashi et al. (2014), which were carried out under the following project studies “Practical technology development project for promoting new agriculture, forestry and fisheries policies” and “Promotion of scientific and technical research in agriculture, forestry, fisheries and food industry” commissioned by the Ministry of Agriculture, Forestry and Fisheries (MAFF).
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interests for this article.




