Effect of bittern immersion on textural properties and water-holding capacity in beef
Abstract
Hard meat has low market value; hence, we used bittern as a novel meat tenderizer for bovine M. semitendinosus, one of a hard muscle. We investigated the effects of beef immersion in bittern, a basic solution primarily comprising MgCl2, on textural properties and water-holding capacity. Muscle samples from M. semitendinosus of Holstein steers were immersed in seven different solutions (RO, NaCl, MgCl2, red wine, pH 3, bittern, and pH 8) and heated at 80°C for 5min. The pH of the beef and immersion solutions, water-holding capacity, and maximum load of the meat were measured. Although beef immersed in red wine (pH 3) had a lower pH and water-holding capacity, that immersed in bittern (pH 8.4) had a higher pH and higher water holding capacity. These results indicate that immersion in acidic red wine may harden beef and that immersion in basic bittern may be more effective in maintaining water-holding capacity and softening beef.
1 INTRODUCTION
The hardness of meat is one of the most important textural characteristics experienced while eating (Ionescu et al., 2008). Hard meat, in general, tends to have low market value due to low evaluation from consumers (Huffman et al., 1996).
Research on meat tenderization has involved several chemical and physical methods, including the use of proteolytic enzymes from plants (Takada et al., 2018, 2019; Takada & Muramoto, 2017). Uda (1966) reported that the water-holding capacity of meat is the lowest at approximately pH 5, and increases in more acidic and basic conditions. Additionally, previous reports demonstrate a negative correlation between juiciness and firmness, and juiciness and elasticity in meat texture (Ruiz de Huidobro et al., 2001). Consequently, soaking meat in acidic or basic solutions can improve its water-holding capacity and tenderness.
Soaking meat in red wine, which is acidic in nature, results in a decrease in pH on account of the presence of organic acids, a concomitant improvement in water-holding capacity, and an improvement in meat tenderness (Nagao, 2014). However, bittern, which is a designated food additive under the Japanese Food Sanitation Act, is basic in nature. It is a concentrated liquid formed by the precipitation of MgCl2 in boiling seawater. The liquid concentrate has a final concentration of 1.0–5.0% magnesium (MgCl2), a key component in the formation of bones and teeth (Haga et al., 2005). Bittern by itself is of little commercial use, apart from its utilization as a coagulant in tofu. Nayak et al. (1996) reported better extraction of myosin and actin from turkey thigh muscle in a NaCl and MgCl2 solution than that in a NaCl solution alone. Notably, the exact effects of immersion in bittern on the water-holding capacity and texture of meat are unknown.
In the present study, we explored the possibility of using bittern as a novel meat tenderizing substance by examining the pH, water holding capacity, and texture of bovine M. semitendinosus, a typically hard to access muscle. The assessment involved examination of the effects of MgCl2 containing bittern and a basic solution with the pH adjusted to that of bittern, as well as red wine and an acidic solution with the pH adjusted to that of the wine.
2 MATERIALS AND METHODS
2.1 Muscle samples
Semitendinosus muscle was excised from five store bought Holstein steers (19.2 ± 0.5 months old). Each excised muscle was stored at 2°C for approximately 48 h post slaughter and vacuum-packed for 10.4 ± 0.8 days at 5°C. Each muscle was subsequently stored at −60°C until further analysis, when it was thawed at 5°C for 48 h.
2.2 Preparation of soaking solution
The pH 3–8 solutions were prepared using buffer solutions, the pH of which was adjusted using citric acid monohydrate (Wako Pure Chemical Corporation, Osaka, Japan) and disodium hydrogen phosphate (Wako Pure Chemical Corporation). The concentration of the NaCl solution (Fujifilm Wako Pure Chemicals Corporation, Osaka, Japan) was set at 0.85%, which was the same as that of the tested bittern (Amami's bittern; Akahokasei Corporation, Hyogo, Japan). NaCl solutions in the pH 3 and pH 8 solutions, and red wine (Derica Maison; Suntory Holdings Limited, Tokyo, Japan), were prepared similarly. The concentration of the MgCl2 solution (Fujifilm Wako Pure Chemicals Corporation, Osaka, Japan) was set at 2.60%, which was the same as that of the tested bittern. Each solution was stored at 5°C until further use.
2.3 Measurement of pH
The pH of the muscle samples before immersion, after immersion, and after heating in hot water was measured using a pH meter of the sticking electrode type (pHspear; Thermo Fisher Scientific Inc., Waltham, MA, USA). The pH of the solutions pre and post immersion were measured using a tabletop pH meter (S220-Basic; METTLER TOLEDO Corporation, Tokyo).
2.4 Physical and chemical analyses
From thawed semitendinosus muscle, 30 × 50 × 5 mm specimens were cut parallel to the muscle fibers so that the muscle fiber cross-section appeared on the 30 × 5 mm surface. Total 96 semitendinosus muscle from five Holstein steers were randomly distributed into eight groups. These were weighed and placed each of the 12 samples in vacuum-packed bags individually containing either 30 mL of distilled water (distilled water group), NaCl solution (NaCl group), MgCl2 solution (MgCl2 group), red wine (red wine group), pH 3 solution (pH 3 group), bittern (bittern group), or pH 8 solution (pH 8 group) before vacuum packing for 2 days at 5°C. Control samples were not immersed. Two days post immersion, drippings on the muscle surface were removed using paper towels and the samples were weighed, and drip loss was determined from the difference in weight prior to and post immersion. The muscle samples were subsequently placed in vacuum-packed bags and heated in a hot water bath at 80°C for 5 min, followed by cooling in water with cube ice for 10 min to halt further heating. Post cooling, drippings on the muscle surface were removed using paper towels and the samples were reweighed. The cooking loss was determined from the difference in weight before and after heating. The total loss was determined from the difference in weight before immersion and after heating. Measurements of maximum load as an indicator of hardness was conducted using a rheometer (CR-3000EX; Sun Scientific Corporation, Tokyo, Japan) and an adapter (No.9) supplied with the rheometer, and performed by compressing the muscle perpendicular to the direction of the muscle fibers using a load cell of 200 N and a test speed of 60 mm/min. Measurements were performed in duplicate for each sample.
2.5 Statistical analysis
The differences in the average value of each measurement item between the control beef and beef immersed in seven different solutions were analyzed using the Tukey–Kramer's multiple comparison tests. The relationship between total loss and maximum load in the control beef and beef immersed in seven different solutions was analyzed using the simple regression analysis. All statistical tests were performed using 4steps Excel Statistics, 4th edition (OMS Publishing, Saitama, Japan).
3 RESULTS AND DISCUSSION
The effects of immersion in seven different solutions on the pH of the Holstein steer semitendinosus muscle are shown in Table 1. Post immersion, the beef pH was lower in the red wine and pH 3 groups but higher in the pH 8 group. The beef pH post heating was higher in all groups except for that treated at pH 3.
| Control | Solutions | |||||||
|---|---|---|---|---|---|---|---|---|
| RO 2 | RO + NaCl | RO + MgCl2 | Red wine | pH 3 | Bittern3 | pH 8 | ||
| pH after immersion4 | 5.8 | 5.6 | 5.9 | 5.3 | 4.9 | 5.1 | 5.5 | 6.8 |
| pH after heating5 | 6.1 | 6.0 | 6.2 | 5.7 | 5.7 | 4.6 | 5.8 | 7.5 |
- 1 The pH of M. semitendinosus before immersion was 5.8.
- 2 RO is the water passed through reverse osmosis film.
- 3 Bittern is the basic concentrated solution of salts left over after the crystallization of seawater.
- 4 All samples were immersed in RO, RO + NaCl, RO + MgCl2, red wine, pH 3 solution, bittern, or pH 8 solutions at 5°C for 2 days.
- 5 All samples were heated at 80°C for 5 min.
Table 2 shows the pH of seven solutions before and after immersion of the semitendinosus muscle. While the pH of distilled water, NaCl, red wine, and the pH 3 solution were higher post immersion in comparison to that before, that of bittern and the pH 8 solution were lower. Thus, while the immersion of beef in red wine (pH 3), an acidic solution, resulted in lower pH, its immersion in bittern (pH 8.4), a basic solution, resulted in a higher pH.
| Solutions | |||||||
|---|---|---|---|---|---|---|---|
| RO1 | RO + NaCl | RO + MgCl2 | Red wine | pH 3 | Bittern2 | pH 8 | |
| pH before immersion3 | 5.2 | 5.1 | 8.8 | 3.0 | 2.8 | 8.4 | 8.0 |
| pH after immersion | 5.4 | 5.3 | 4.6 | 3.9 | 3.7 | 4.8 | 7.3 |
- 1 RO is the water passed through reverse osmosis film.
- 2 Bittern is the basic concentrated solution of salts left over after the crystallization of seawater.
- 3 All samples were immersed in RO, RO + NaCl, RO + MgCl2, red wine, pH 3 solution, bittern, or pH 8 solutions at 5°C for 2 days.
The effects of immersion in seven different solutions on drip loss, cooking loss, and total loss of the Holstein steer semitendinosus muscle are shown in Table 3. Cooking loss was significantly lower in the control, MgCl2, red wine, bittern, and pH 8 groups than in the distilled water, NaCl, and pH 3 groups (P < 0.05). Drip and total losses were significantly lower in the MgCl2, bittern, and pH 8 groups than that in the control, distilled water, NaCl, red wine, and pH 3 groups (P < 0.05). Thus, while the immersion of beef in red wine (pH 3), an acidic solution, resulted in lower water-holding capacity, its immersion in bittern (pH 8.4), a basic solution, suppressed drip leakage and resulted in higher water-holding capacity.
| Control | Solutions | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RO2 | RO + NaCl | RO + MgCl2 | Red wine | pH 3 | Bittern3 | pH 8 | ||||||||||||||||||
| Drip loss (%) | 4.2 | ± | 0.4a | 2.7 | ± | 3.8a | −3.0 | ± | 1.6a | −25.1 | ± | 3.0b | 8.1 | ± | 0.3a | 5.5 | ± | 1.3 a | −30.7 | ± | 3.5bc | −38.2 | ± | 3.2c |
| Cooking loss (%) | 27.1 | ± | 0.7c | 43.1 | ± | 0.4a | 40.6 | ± | 1.2ab | 27.4 | ± | 1.4c | 30.8 | ± | 0.7c | 37.1 | ± | 0.3b | 29.5 | ± | 0.6c | 21.6 | ± | 0.9d |
| Total loss (%) | 30.4 | ± | 0.5c | 45.5 | ± | 0.5a | 38.8 | ± | 0.8b | 9.4 | ± | 1.7d | 36.4 | ± | 0.5b | 40.6 | ± | 0.6ab | 7.9 | ± | 1.9d | −8.3 | ± | 1.9e |
- 1 Means ± SE (n = 12).
- 2 RO is the water passed through reverse osmosis film.
- 3 Bittern is the basic concentrated solution of salts left over after the crystallization of seawater.
- a–e Means within a row with a different superscript letter differ significantly (P < 0.05).
Rome (1967) reported that actomyosin, a complex of actin and myosin that comprises a major component of myofibrils, is negatively charged due to a lower hydrogen ion concentration in pH more basic than its isoelectric point. The consequent electrical repulsion between actomyosin causes the myofibrils to push each other apart. This accounts for the improved water-holding capacity of beef post its immersion in a basic solution of bittern, where the electrical repulsion between actomyosin allows water flow into the myofibrils. Rome (1967) further reported that actomyosin is positively charged due to a higher concentration of hydrogen ions in pH more acidic than its isoelectric point, which also results in electrical repulsion between actomyosin molecules that push myofibrils apart. Mega et al. (1980) reported that endogenous acidic proteases degrade myosin when meat is immersed in an acetic acid solution. However, Nagao (2014) reported soaking meat in red wine, which is acidic in nature, results in a decrease in pH on account of the presence of organic acids, a concomitant improvement in water-holding capacity, and an improvement in meat tenderness. Therefore, it is considered that further investigation is necessary to reveal the reason of the water-holding capacity of beef immersed in red wine could be higher or lower.
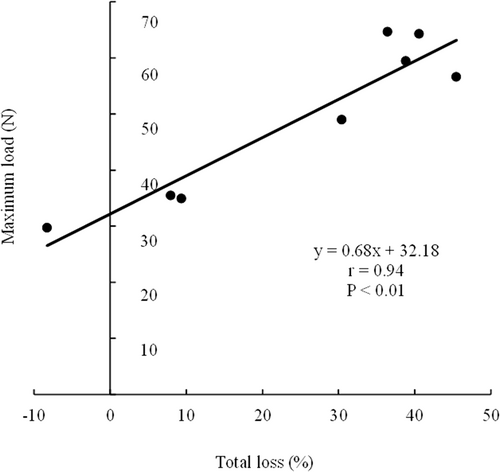
The effects of immersion in seven different solutions on the maximum load on Holstein steer semitendinosus muscle are shown in Table 4. The maximum load was significantly lower (P < 0.05) in the MgCl2, bittern, and pH 8 groups than that in the control, distilled water, NaCl, red wine, and pH 3 groups. The maximum loads in the red wine and pH 3 groups were significantly higher (P < 0.05) than those in the control group. Further, there were no significant differences (P > 0.05) between the maximum loads in the red wine and pH 3 groups and those in the distilled water and NaCl groups. Thus, the immersion of beef in acidic red wine (pH 3) hardens the beef, whereas immersion in basic bittern (pH 8.4) softens it. Önenç et al. (2004) reported that meat hardens at pH below its isoelectric point, and Sheard and Tali (2004) demonstrated the toughening of chicken breast meat on immersion in an acidic solution, which is consistent with our findings. Figure 1 shows the relationship between total loss and maximum load in the control beef and beef immersed in seven different solutions. We found a significant positive correlation between total loss and maximum load (P < 0.001, r = 0.94). This result suggests that the hardness of the meat increases as the water-holding capacity decreases. Meat with higher water retention is softer (Goli et al., 2014), which may account for the softening of meat observed in this study post immersion in the basic solution. The hardening of beef post immersion in acidic solutions in this study may have been more of a consequence of reduced water-holding capacity than the effect of myofibrillar degradation.
| Control | Solutions | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RO2 | RO + NaCl | RO + MgCl2 | Red wine | pH 3 | Bittern3 | pH 8 | ||||||||||||||||||
| Maximum load (N) | 49.0 | ± | 0.6b | 56.6 | ± | 2.7ab | 59.5 | ± | 4.1ab | 34.9 | ± | 0.9c | 64.7 | ± | 3.4a | 64.3 | ± | 2.4a | 35.5 | ± | 1.9c | 29.7 | ± | 2.8c |
- 1 Means ± SE (n = 12).
- 2 RO is the water passed through reverse osmosis film.
- 3 Bittern is the basic concentrated solution of salts left over after the crystallization of seawater.
- a-c Means within a row with a different superscript letter differ significantly (P < 0.05).
We observed no difference in water-holding capacity and maximum load between the control and NaCl groups. Medyński et al. (2000) reported that bovine biceps femoris (M. biceps femoris) had greater water-holding capacity post immersion in NaCl concentrations above 1.5%. We set our NaCl concentration at 0.85%, which is equal to that of bittern, to account for differences in osmotic pressure. This concentration of NaCl; however, did not seem to have any effect on meat softening. In contrast, while the water-holding capacity in the MgCl2 group was higher than that in the control group, the maximum load was lower in the MgCl2 group than that in the control group. This suggested that meat was softened at the same MgCl2 concentration (2.60%) as that of the bittern.
The water-holding capacity of meat is the lowest at approximately pH 5, and more acidic and basic solutions have higher water holding capacities. We, however, observed the hardening of beef post immersion in acidic wine. Thus, immersion in basic bittern that primarily comprises MgCl2 may maintain water-holding capacity and tenderize meat more effectively.
ACKNOWLEDGMENTS
The authors express their deep gratitude to the Aomori Prefectural Industrial Technology Research Center Food Processing Station for their cooperation in this study. We would like to thank Editage (www.editage.jp) for English language editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest for this article.




