Denatured collagen in keratin layers and smooth muscles of teats with low or high teat apex scores in Holstein dairy cows
Abstract
We hypothesized that teats with a teat apex score (TAS) of 4 on a 4-point scale would exhibit elevated levels of denatured collagen compared with teats with lower TAS. We procured keratin layer and smooth muscle samples from Holsteins with TAS ranging from 1 to 4, as well as from crossbred heifers (Japanese Black male and Holstein female) with TAS of 1. Teats with a TAS of 4 demonstrated increased total collagen content, higher amounts of type I collagen (the harder, thicker variant), and reduced amounts of type III collagen (the softer, thinner variant) compared with teats with lower TAS. Teats with TAS of 3 and 4 exhibited evidence of damaged collagen in smooth muscle layers compared with teats with TAS of 1. Additionally, we identified 47-kDa heat shock protein-positive fibroblasts in the smooth muscles of teats with TAS of 3 and 4. Therefore, the smooth muscle of teats with a TAS of 4 exhibited increased amounts of denatured collagen in comparison to teats with lower TAS.
1 INTRODUCTION
Mastitis is a global issue in dairy farming. The teat canal (Ductus papillaris mammae) is a body opening enveloped by a keratin layer (from Stratum corneum to Stratum basale) and encircled by smooth muscles that facilitate opening and closure (van der Merwe, 1985). The onset of mastitis in dairy cows is attributed to pathogenic bacteria infiltrating the teat canal (Paulrud, 2005). Various factors associated with machine milking contribute to the deterioration of teat condition (Odorčić et al., 2019). The assessment of the teat apex score (TAS) on a 4-point scale proves to be a valuable management tool (Zecconi et al., 2005). Notably, dairy cows with elevated TAS levels are more susceptible to mastitis (Sharma et al., 2016). However, scant information exists regarding disparities in the keratin and smooth muscle layers among varying TAS levels.
Collagen stands out as one of the most abundant proteins in the extracellular matrix and plays a crucial role in the primary defense against external pathogens (Chambers & Vukmanovic-Stejic, 2020). The denaturation of collagen is increased due to various diseases, including cancer, osteoporosis, and arthritis (Fields, 2013; Ito & Nagata, 2019). The technical challenges of detecting damaged collagen in tissues were recently overcome with the development of a biotin-labeled collagen-mimetic peptide capable of hybridizing with the denatured portion of collagen (Takita et al., 2019). Utilizing this denatured collagen detection reagent, we have recently pioneered an in situ detection method to identify areas rich in denatured collagen in bovine oviducts, uteri, and cervices (Ferdousy et al., 2023; Ferdousy & Kadokawa, 2022), though not yet applied to teats.
The 47-kD heat shock protein, HSP47, encoded by SERPINH1, stands as the exclusive procollagen-specific molecular chaperone crucial for the accurate folding of collagen's unique triple-helical structure (Ito & Nagata, 2019). HSP47 also assumes a pivotal role in collagen synthesis and the prevention of procollagen aggregation (Duarte & Bonatto, 2018). Consequently, we have recently employed HSP47 as a marker to pinpoint the location of active collagen synthesis in bovine oviducts and uteri (Ferdousy et al., 2023; Ferdousy & Kadokawa, 2022). While HSP47 serves as a key regulator of cell homeostasis, excessive expression is implicated in collagen-related diseases, such as keloids and fibrosis (Ito & Nagata, 2019). HSP47 has been identified on the surface epithelial cells of lungs afflicted with pneumonia (Kakugawa et al., 2005) and has been implicated in inducing mesenchymal phenotypes in mammary epithelial cells during cancer metastasis (Xiong et al., 2020).
In this study, employing an in situ detection method for damaged collagen and immunohistochemistry for HSP47, we sought to test the hypothesis that the levels of denatured collagen and HSP47 in the keratin layers and smooth muscle of teats with a TAS of 4 would surpass those in teats with lower TAS.
2 MATERIALS AND METHODS
2.1 Ethics approval
All experiments were performed according to the Guiding Principles for the Care and Use of Experimental Animals in the Field of Physiological Sciences (Physiological Society of Japan) and were approved by the Committee on Animal Experiments of Yamaguchi University (approval no. 301).
2.2 Sample collection
Teat samples were acquired from cattle under the care of contract farmers in western Japan. Cows were in mid- or late-lactation periods. Cows and heifers were accommodated in free barns and were provided with a total mixed ration comprising grass silage (mainly Italian ryegrass), rice silage, flaked corn, and soybean meal in accordance with the Japanese Feeding Standards for Dairy Cattle (Agriculture, Forestry and Fisheries Research Council Secretariat, 2017). Water and mineral blocks (Koen-S, Nippon Zenyaku Kogyo, Co., Ltd., Fukushima, Japan) were freely available. All cattle born in Japan since 2003 are registered at birth with an individual identification number in the National Livestock Breeding Center of Japan's database. We utilized these individual identification numbers, along with information provided by contract farmers, for the cattle in this study.
We also collected keratin or smooth muscle samples to perform reverse transcription-polymerase chain reaction (RT-PCR) or western blot using the methods described in Figure 1. Briefly, the teats were recovered within 5 min after slaughter, and the epidermal layers (surface skin) and unnecessary portions were carefully dissected. Then, we collected the keratin layer facing the canal and the smooth muscle layer. The entire tissue fragment was approximately 5 mm wide, 5 mm long, and 2 mm thick.
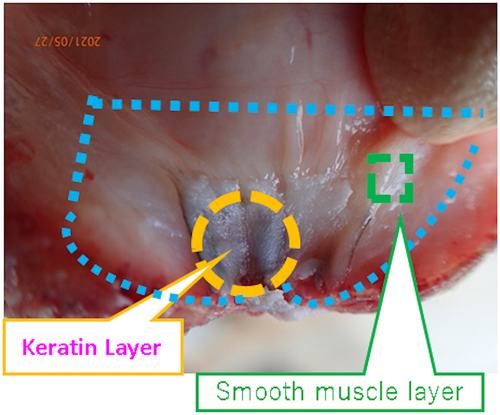
2.3 HE staining
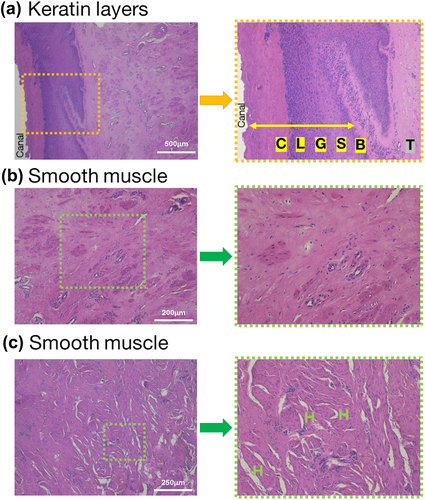
Tissues were fixed, dehydrated, and paraffin-embedded using an automated tissue processor (ASP6025; Leica Microsystems, Wetzlar, Germany). Thin sections (5 μm thick) were cut with a microtome (RM2245; Leica Microsystems) and affixed to slide glass (MAS coat Superfrost, Matsunami-Glass, Osaka, Japan). The paraffin-embedded sections underwent deparaffinization in xylene and dealcoholized in ethanol and ultrapure water for 5 min each. The slides were then covered in hematoxylin (New Type M; Muto Pure Chemicals Co. LTD., Tokyo, Japan) for 5 min. After washing, eosin (New Type M; Muto Pure Chemicals Co. LTD.) was used for staining for 3 min; then, the sections were dehydrated in 70%, 90%, 100%, and 100% ethanol and cleared with three changes of xylene. After attaching the coverslip with the Entellan new mounting medium (Sigma-Aldrich, St. Louis, MO, USA), the stained sections were observed under a bright-field light microscope (Eclipse Si; Nikon, Tokyo, Japan) with a digital camera (MC120 HD; Leica Microsystems). Teat layers were differentiated based on prior studies (Liebich et al., 2019; Paulrud, 2005), as shown in Figure 2. We defined “hole” as a continuous line or polygonal empty, but not blood vessels.
2.4 Picrosirius red staining for total collagen
A previously reported picrosirius red staining method was employed to detect total collagen (Ferdousy et al., 2023; Ferdousy & Kadokawa, 2022). The same protocol was applied to prepare paraffin-embedded sections (5 μm thick). After deparaffinization, slides were covered in picrosirius red staining solution (Picro-Sirius Red Stain Kit; ScyTek Laboratories Inc., Logan, UT, USA) for 1 h. Following staining, slides were washed with 0.5% acetic acid solution, dehydrated in ethanol, and cleared in xylene. After coverslip attachment, sections were observed under both bright field and polarized light using the same microscope system. Bright-field microscopy revealed dense collagen regions in dark red, collagen-less regions in yellow, and intermediate regions in orange (de Oliveira et al., 2013). Polarized light microscopy displayed type I collagen (the harder, thicker type) in red or yellow and type III collagen (the softer, thinner type) in green (de Oliveira et al., 2013).
2.5 In situ detection of denatured collagen and immunofluorescence staining
We employed previously established in situ detection methods for denatured collagen immunofluorescence staining (Ferdousy et al., 2023; Ferdousy & Kadokawa, 2022). Initially, unfixed tissue blocks were sectioned into 10-μm-thick slices using a cryostat (CM1950; Leica Microsystems) and mounted on slides. The tissue was then fixed with 4% PFA in PBS for 15 min. Blocking was achieved by incubating the tissue sections in PBS (0.5 mL) containing 10% normal goat serum (Wako Pure Chemicals, Osaka, Japan) for 1 h at room temperature. Subsequently, the tissue sections underwent treatment with an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA, USA) following the manufacturer's protocol. After washing twice with PBS, the slide was loaded with 5 μg/mL of denatured collagen detection reagent (Funakoshi, Tokyo, Japan) and incubated for 60 min at room temperature in a humid box. After two washes with PBS, the tissues were incubated with streptavidin, Alexa Fluor 546 conjugate (diluted to 1 μg/mL in PBS; Thermo Fisher Scientific, Waltham, MA, USA) for 60 min at room temperature.
Following three PBS washes, the sections were treated with 0.3% Triton X-100 for 15 min, blocked with 10% normal goat serum in PBS for 1 h at room temperature, and subjected to a primary antibody reaction with the previously reported anti-HSP47 rabbit polyclonal antibody for immunohistochemistry (Ferdousy et al., 2023; Ferdousy & Kadokawa, 2022) (1:1000 dilution; AP7366B; Abcepta Inc., San Diego, CA, USA) overnight at 4°C. Subsequently, the samples underwent a secondary antibody reaction with Alexa Fluor 647 goat anti-rabbit IgG (diluted to 1 μg/mL, Thermo Fisher Scientific) and 1 μg/mL of 4, 6-diamino-2-phenylindole (DAPI; Wako Pure Chemicals) for 2 h at room temperature. After washing the sections four times, the slides were mounted in preparation for confocal microscopy. To verify signal specificity, several negative controls were included, such as omission or preabsorption of the primary antiserum with 5-nM antigen peptide, substitution of normal rabbit IgG (Wako Pure Chemicals) for the primary antibody, omission of secondary antibodies, and use of normal goat IgG (Wako Pure Chemicals) instead of secondary antibodies. Additionally, negative controls involved preabsorption of secondary antibodies with 5-nM normal rabbit IgG.
The sections were observed using a confocal microscope (LSM710; Carl Zeiss, Göttingen, Germany) equipped with a 405-nm diode laser, 488-nm argon laser, 533-nm HeNe laser, and 633-nm HeNe laser. Fluorescence microscopy images were captured with a 20× or 40× oil-immersion objective and recorded using a charge-coupled device (CCD) camera system controlled by ZEN2012 black edition software (Carl Zeiss). Consistent microscope settings were maintained for immunofluorescence imaging of the samples to facilitate group comparisons.
2.6 Crushing frozen teat samples into powder
Keratin or smooth muscle samples (n = 5 per group) were frozen in liquid nitrogen and subsequently transferred into cooled 3 mL vials (ST-0320PCF; Yasui Kikai Co. Ltd., Osaka, Japan). Cooled metal cones (MC-0316; Yasui Kikai Co. Ltd.) were input into the vials, which underwent freeze-crushing (2700 rpm, 20 s, one cycle) using a multisample holder (MSH003; Yasui Kikai Co. Ltd.) attached to a multibead shocker (MB1001CS; Yasui Kikai Co. Ltd.). The samples were crushed into powder and utilized for RNA or protein extraction.
2.7 Reverse transcription-polymerase chain reaction, sequencing of amplified products, and homology search in gene databases
Following the immediate crushing of samples, RNAzol RT isolation reagent (Molecular Research Centre Inc., Cincinnati, OH, USA) was added to the vials and thoroughly mixed for total RNA extraction. Postprocessing in accordance with the manufacturer's protocol, the concentration and purity of each RNA sample were assessed via spectrophotometry. After removing potentially contaminated genomic DNA with deoxyribonuclease, the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) was employed for complementary deoxyribonucleic acid (cDNA) synthesis. No reverse transcription controls (NRCs) were prepared for RT-PCR; they were generated by treating the extracted RNA with the same deoxyribonuclease but excluding cDNA synthetase.
To confirm the presence of HSP47 mRNA (NCBI reference sequence of bovine HSP47 is NM_001046063), PCR was executed using previously reported primers (Ferdousy et al., 2023; Ferdousy & Kadokawa, 2022): nucleotides 770–1239, forward primer: 5′-GACAACCGAGGCTTCATGGT-3′ (exon 4); reverse primer: 5′-AGCTCCTCACGCCCGTAGAT-3′ (exon 6). The expected PCR product size for HSP47 with the primer pair is 470 bp. PCR was carried out using 20 ng of cDNA, or 20 ng of RNA as the NRC, and polymerase (Ex-Premier DNA Polymerase, Takara Bio Inc., Shiga, Japan) under the following thermocycles: 94°C for 1 min for predenaturing followed by 35 cycles of 98°C for 10 s, 60°C for 15 s, and 68°C for 30 s. Separation of PCR products was done on a 1.5% agarose gel through electrophoresis with a molecular marker (Nippon Gene, Tokyo, Japan), stained with Gelstar (Lonza, Allendale, NJ, USA), and observed using a CCD imaging system (FAS-BG Led box, Nippon Genetics, Tokyo, Japan). The PCR products were purified with the NucleoSpin Extract II kit (Takara Bio Inc.) and subsequently sequenced using one of the PCR primers and the Dye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific). The obtained sequences were utilized as query terms for a homology search using BLAST (available on the NCBI website).
2.8 Western blotting for HSP47 detection
Immediately after crushing, a tissue protein extraction reagent (Thermo Fisher Scientific) containing a Halt protease inhibitor cocktail (Thermo Fisher Scientific) was added to the vials and mixed for protein extraction. The total protein content of each tissue homogenate was estimated using the bicinchoninic acid kit (Thermo Fisher Scientific). The extracted protein samples were boiled with sample buffer solution with reducing reagent (6x) for SDS-PAGE (09499-14; Nacalai Tesque, Kyoto, Japan) for 3 min at 100°C. Protein samples (8000 ng of total protein) were loaded onto a sodium dodecyl sulfate–polyacrylamide gel (4%–15% Criterion TGX gel, Bio-Rad, Hercules, CA, USA) alongside a molecular weight marker (Multicolor Protein Ladder; Nippon Gene Co. Ltd., Tokyo, Japan). Keratin layer and smooth muscle samples were electrophoresed separately due to the gel having only 26 wells. Gels ran at 100 V for 90 min. Proteins were transferred onto polyvinylidene fluoride (PVDF) membranes via electroblotting at 2.5 A, 25 V, for 7 min using the Trans-blot Turbo system (Bio-Rad). The PVDF membrane was stained with Revert 700 total protein stain (LI-COR Biosciences, Lincoln, NE, USA) for 15 min. After two 30-s washing cycles with 30% methanol containing 6.7% acetic acid, the PVDF membrane was neutralized with 10-mM Tris–HCl (pH 7.6) and 150-mM NaCl. Subsequently, the membrane was scanned using Odyssey CLx (LI-COR Biosciences).
The Can Get Signal Immunoreaction Enhancer kit (Toyobo Co. Ltd, Osaka, Japan) served as a blocking membrane (1 h at 25°C) for the primary antibody reaction (16 h at 4°C) with the anti-HSP47 antibody (1:400,000 dilution in 20 mL of immunoreaction enhancer solution I supplemented with 30-μg normal goat IgG) and secondary antibody reaction (1 h at 25°C) with goat anti-rabbit IgG horseradish peroxidase-conjugated antibody (Bethyl Laboratories Inc., Montgomery, TX, USA; 1:400,000 dilution in 20 mL of immunoreaction enhancer solution II supplemented with 30-μg normal goat IgG).
Protein bands were visualized using the Amersham ECL-Prime chemiluminescence kit (Cytiva, Marlborough, MA, USA) and a CCD imaging system (Amersham Image Quant 800, Cytiva). To verify signal specificity, several negative controls were included, wherein the primary antibodies were omitted or normal rabbit IgG were used instead of the primary antibodies. Signal specificity was also confirmed using negative controls in which the primary antibodies were preabsorbed with 5 nM of antigen peptide (Scrum Inc., Tokyo, Japan). ImageQuant TL (version 8.2; Cytiva) software was used to measure the band sizes and volumes (calculated using rolling ball background subtraction).
3 RESULTS
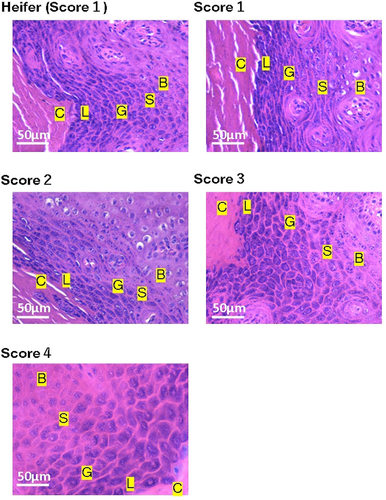
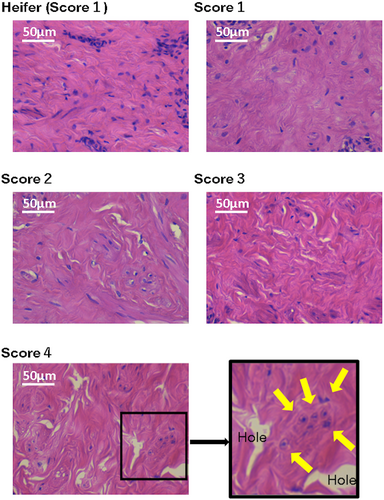
3.1 HE staining
Figures 3 and 4 illustrate examples of the HE-stained keratin layer and smooth muscle in the teats of heifers and cows. We observed holes in the teat smooth muscles with a TAS of 2 and more numerous holes in the smooth muscles of the teats with TAS of 3 and 4. Fibroblasts were noted in proximity to these holes.
3.2 Picrosirius red staining for total collagen
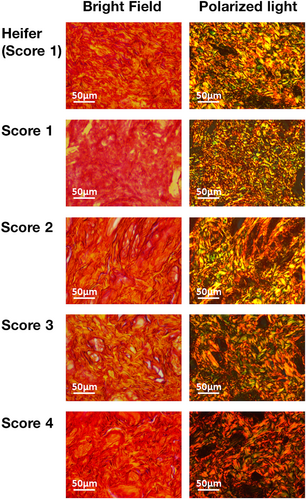
Picrosirius red staining, coupled with bright-field microscopy, displayed red or orange hues throughout the keratin layer (Figure 5) and smooth muscle (Figure 6).
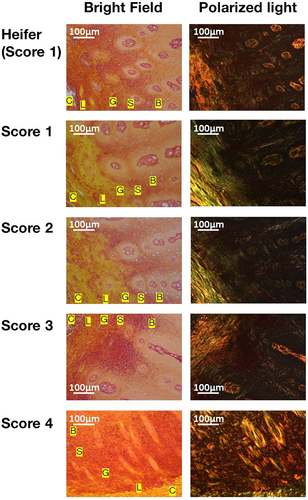
In the keratin layers, the teats with TAS of 1 showed orange in the stratum corneum, stratum lucidum, stratum granulosum, and stratum spinosum. In contrast, the keratin layers of teats with TAS of 4 exhibited more dark red regions compared with other scores, which predominantly displayed orange regions. Polarized light microscopy showed green regions in the stratum corneum, stratum lucidum, and stratum granulosum. In contrast, an abundance of red or yellow regions in teats with TAS of 4, contrasting with more prevalent green regions in other scores.
In smooth muscle, teats with a TAS of 1 also showed bright red. In contrast, the keratin layers of teats with a TAS of 4 showed more dark red regions compared with other scores. Polarized light microscopy showed green or yellow fibers in teat smooth muscle with a TAS of 1. In contrast, polarized light microscopy showed an abundance of red fibers in teats with TAS of 4.
3.3 Reverse transcription-polymerase chain reaction
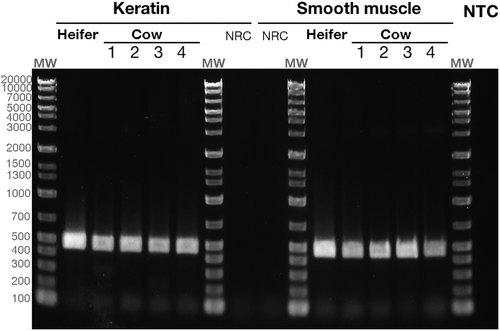
PCR products corresponding to the size of HSP47 (470 bp) were obtained from both keratin layers and smooth muscle, as evidenced by agarose gel electrophoresis (Figure 7). Non-reverse transcription controls (NRCs) did not yield any PCR-amplified products. A homology search against gene databases for the sequenced amplified products identified bovine HSP47 (NM_001046063) as the best match, with 100% query coverage, an e-value of 0.0, and a maximum alignment identity of 99%. No other bovine genes exhibited homology with the PCR product, confirming its identity as HSP47.
3.4 Western blotting for HSP47 detection
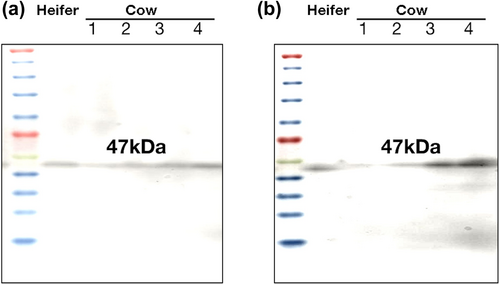
Western blotting confirmed the presence of HSP47 protein in all specimens from teats with TAS of 1–4 (Figure 8). Strong bands were detected in the smooth muscles of teats with TAS of 4. In contrast, only weak bands were detected in the keratin layers of all groups.
3.5 In situ detection of denatured collagen and immunofluorescence staining
In the keratin layers (Figure 9), teats with a TAS of 1 showed only a weak HSP47 signal in the epithelial layer and in the superficial stroma near the epithelial layer. The teats with a TAS of 1 showed no signals of denatured collagen. In contrast, the keratin layers of teats with TAS 3 and 4 showed robust, high-intensity fluorescence signals of denatured collagen and HSP47 signals in the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale.
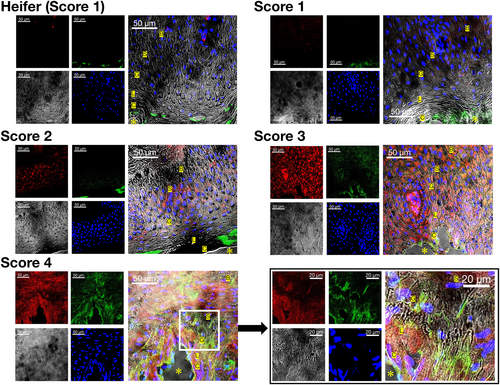
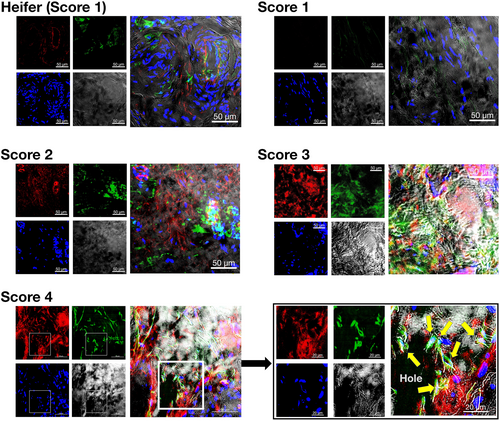
In smooth muscle (Figure 10), teats with a TAS of 1 showed only a weak HSP47 signal and no denatured collagen signals. In contrast, the smooth muscle of teats with TAS 3 and 4 showed robust, high-intensity fluorescence signals of denatured collagen and HSP47 signals. Notably, many holes in the smooth muscles of teats with TAS of 3 and 4 were observed, with concurrent identification of HSP47-positive fibroblasts.
4 DISCUSSION
The teat canals of cows necessitate precise opening and closure orchestrated by smooth muscle power, yet the intricate molecular and cellular mechanisms governing these processes remain largely unexplored. Smooth muscles, crucial for maintaining the mechanical properties of human arteries (Bank et al., 1996), rely on associated collagen. Collagen, a key determinant of tissue tensile strength (Sharma et al., 2022), was ubiquitously present in the keratin layer and smooth muscle of the teats, as evidenced by our picrosirius red stain. Teats with TAS of 4 exhibited heightened levels of thick, rigid collagen alongside damaged collagen. Consequently, high-TAS teats showcased abnormal collagen patterns, implying a potential disorder in teat canal functioning for timely opening and closure. This abnormality elevates the risk of pathogenic microorganism infiltration.
While collagen and HSP47 levels in teats across species remain poorly understood, limiting direct comparisons with prior studies, machine milking emerges as a probable culprit in teat damage, particularly through inappropriate vacuum systems, machine liner slips, and overmilking (Besier & Bruckmaier, 2016; Odorcic et al., 2020; Rogers & Spencer, 1991). Teat-end callosity rapidly increases postpartum in dairy cows, persisting for over 3 months (Neijenhuis et al., 2000). Post-injury, dermal fibroblasts migrate and transdifferentiate into myofibroblasts, contributing to teat-end callosity thickness maintenance.
In contrast to non-denatured type I collagen, which aids sunburned skin healing (Fu et al., 2022), denatured type I collagen induces fibroblast differentiation into myofibroblasts and promotes cell survival in vitro (Su et al., 2022). This denatured collagen might originate directly from myofibroblasts, suggesting a dynamic process beyond simple denatured collagen fibers (Kaku et al., 2023). Moreover, proteolytic collagen degradation yields bioactive fragments known as matricryptins or matrikines, regulating inflammatory, reparative, and fibrogenic cascades in acute and chronic conditions (de Castro Brás & Frangogiannis, 2020). Fibroblasts play a major role in the production of matricryptins, which exert fibrogenic or reparative actions by modulating fibroblast phenotype and function (de Castro Brás & Frangogiannis, 2020). Therefore, the denatured collagen-rich region may be a matricryptin-production sites affecting diverse regulatory processes.
HSP47 is induced by cellular stress, but it is also constitutively expressed, and its expression is always upregulated or downregulated concomitantly with changes in the expression of various types of collagen (Ito & Nagata, 2017). Therefore, we speculated that both single-positive and co-positive cells for HSP47 and denatured collagen might be myofibroblasts. HSP47 binds to the ubiquitin-like protein UBIN (Matsuda et al., 2001) and undergoes degradation through the ubiquitin–proteasome system (Ito & Nagata, 2016). Investigations are needed to explore whether HSP47 protein degradation in teats, shortly after translation, occurs smoothly in high-TAS teats, resulting in abnormal collagen synthesis.
Collagen and HSP47 expression were observed in heifers' teats, suggesting the need to prevent microbial invasion without milk ejection. As heifers' body size increases, teat size grows with enhanced extracellular matrix synthesis (Inoue et al., 2020). Ovarian steroid hormones drive extracellular matrix remodeling in the bovine oviduct (Gonella-Diaza et al., 2018), and we previously found consensus response element sequences for estrogen and progesterone in the 5′-flanking region of the bovine SERPINH1 gene (Ferdousy & Kadokawa, 2022). Changes in collagen amount and quality may occur as teats develop from heifers to lactating cows due to hormonal fluctuations during growth, pregnancy, and lactation.
Takita et al. (2019) established a standard curve based on an enzyme immunoassay using denatured collagen detection reagent and heated pure collagen (grade for culture dish coating). However, our attempts at immunoassays (HRP-conjugated streptavidin for enzyme immunoassay, fluorochrome-conjugated streptavidin for fluorescent immunoassay, lanthanide-conjugated streptavidin for dissociation-enhanced lanthanide fluorescence immunoassay) failed to obtain a good parallel between the standard curve and serially diluted bovine teat extracts that were obtained using various methods. This was because the samples showed strong matrix effects, unlike pure collagen. Therefore, our comparison could only be performed on photographic images. Additionally, we did not quantify the area of denatured collagen using the image, because the staining method is qualitative and not quantitative, as is fluorescent immunohistochemistry.
In conclusion, denatured collagen was higher in teats with TAS of 4 compared with those with lower TAS. Further research is needed to devise new therapies based on recent findings in human medicine.
ACKNOWLEDGMENTS
Yvan Bienvenu Niyonzima was supported by a scholarship from Japan International Cooperation Agency (Tokyo, Japan). This work was partly supported by a Grant-in-Aid for Scientific Research (JSPS Kakenhi grant number 21H02345) from the Japan Society for the Promotion of Science (Tokyo, Japan) to Hiroya Kadokawa.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest for this article.




