Effects of bisphenol A and tauroursodeoxycholic acid on maturation of porcine oocytes and parthenogenetic development of embryos
Ling Yang and Qiongao Zhang contributed equally to this work.
Abstract
Prolonged exposure of bisphenol A (BPA) has adverse effects on in vitro maturation (IVM) of oocytes, but treatment with tauroursodeoxycholic acid (TUDCA) can improve the IVM and development of embryos. The purpose of this study was to investigate the effects of BPA and both BPA and TUDCA on IVM and parthenogenetic development of embryos. The results showed that BPA treatment adverse effects on the cumulus expansion index, survival rate, polar body rate, mitochondrial distribution of the oocytes after maturation culture, and that it also decreased the cleavage rate and blastocyst rate of embryos after parthenogenetic develpoment. In addition, BPA treatment upregulated expression of genes related to endoplasmic reticulum stress and apoptosis and increased the intracellular reactive oxygen species (ROS) level, while it decreased expression of genes related to cumulus expansion. However, the supplementation of TUDCA relieved these adverse effects of BPA except polar body rate, blastocyst rate, and expression of BCL2 and PTGS1. In conclusion, the supplementation of TUDCA can partly attenuate the negative effects of BPA on IVM and parthenogenetic development of embryos, possibly by modification of the expression of genes related to endoplasmic reticulum stress, apoptosis and cumulus expansion, intracellular ROS level, and mitochondrial distribution.
1 INTRODUCTION
Bisphenol A (BPA) is a synthetic chemical used in the production of polycarbonates and epoxy resins, and plastic waste releases BPA into the environment. It has been proved that BPA is an endocrine disruptor and prolonged exposure of BPA has adverse effects on human and animal health (Abraham & Chakraborty, 2020). The adverse effects of BPA include carcinogenesis, reproductive toxicity, abnormal inflammatory or immune response, and developmental disorders of brain or nervous system in living organisms (Murata & Kang, 2018). BPA impairs the morphology and function of organs related to female reproduction and disrupts estrous cyclicity and implantation, which weakens female normal reproductive capacity (Ziv-Gal & Flaws, 2016). BPA induces oxidative stress, impairs redox homeostasis, and causes mitochondrial dysfunction, which has negative effects on female reproductive functions (Meli et al., 2020). Endoplasmic reticulum (ER) stress is induced by 100 μM BPA in non-parenchymal hepatocytes, which is due to the production of intracellular reactive oxygen species (ROS) (Asahi et al., 2010).
ER performs various functions in cells, including synthesis of proteins for degradation of xenobiotics, but bioaccumulation of drugs/chemicals/xenobiotics causes ER stress. ER stress-related genes include glucose-regulated protein 78 (GRP78) and activating transcription factor 4 (ATF4) (Rana, 2020). Monocrotaline induces ER stress in hepatocytes, which changes expression of ER stress-related proteins, including GRP78, ATF4, and C/EBP homologous protein (CHOP) (Guo et al., 2021). ER stress induces apoptosis, and CHOP plays an essential role in ER stress-induced apoptosis (Hu et al., 2019). Activation of the Akt pathway improves expression of anti-apoptotic proteins, like B-cell lymphoma 2 (BCL2), while CHOP can inhibit BCL2 expression but upregulate cleaved caspase-3 (CASP3) and lead to cell death (Tao et al., 2017). Palmitic acid promotes expression of ER stress marker genes (GRP78 and CHOP) and enhances apoptosis-related gene expression (CASP3 and BCL2-associated X [BAX]) in human osteoblast-like Saos-2 cells (Yang et al., 2018).
BPA has been detected in the human follicular fluid, and BPA exposure weakens oocyte in vitro maturation (IVM) in a dose-dependent manner, which has negative effects on oocyte meiotic maturation, spindle morphology, and chromosome alignment in humans (Machtinger et al., 2013). The developmental capacity of oocyte is significantly decreased under exposure of 100 μM BPA, which accompanies with ER stress and upregulation of GRP78 during mouse oocyte maturation (Pan et al., 2021). BPA (75 μM) treatment results in downregulation of meiotic maturation and cumulus cell expansion of cumulus-oocyte complexes (COCs), but upregulation of mitochondrial apoptotic proteins during porcine oocyte maturation (Park, Park, et al., 2018).
Tauroursodeoxycholic acid (TUDCA) acts as a chemical chaperone and can inhibit apoptosis and attenuate ER stress (Amaral et al., 2009). TUDCA treatment can improve blastocyst formation rates after IVM of cumulus-free oocytes in mice (Mochizuki et al., 2018). TUDCA reduces ER stress-induced apoptosis by tunicamycin and enhances the developmental competence of porcine embryos during the pre-implantation stage (Kim et al., 2012). TUDCA improves the maturation rate but reduces ROS in denuded oocytes and decreases ER stress and apoptosis, expression of GRP78/BIP proteins in bovine matured COCs (Khatun, Wada, et al., 2020). TUDCA supplementation (200 μM) decreases ROS production but increases the expression of genes related to antioxidant activity in oocytes and embryos, which is associated with ER stress relief in cattle (Pioltine et al., 2021). TUDCA treatment also improves the developmental competence of porcine somatic cell nuclear transfer embryo through downregulating pro-apoptotic gene BAX but upregulating anti-apoptotic gene BCL2 to attenuate ER-stress and apoptosis (Lin et al., 2016).
The culture system of porcine oocytes could be used for an experimental model to examine reproduction toxicity of BPA. It was hypothesized that BPA treatment had negative effects on the development of in vitro oocyte and embryo, and TUDCA could relieve these negative effects induced by BPA. Therefore, the aim of study was to investigate the effects of BPA and TUDCA on cumulus expansion, oocyte survival rate, polar body rate, mitochondrial distribution, and intracellular levels of ROS of oocytes, as well as cleavage rate and blastocyst rate. In addition, expression of genes related to ER stress (GRP78, ATF4, and CHOP), apoptosis (CASP3, BCL2, and BAX), and cumulus expansion (prostaglandin-endoperoxide synthase 1 [PTGS1], PTGS2, and pentraxin 3 [PTX3]) were analyzed in sows.
2 MATERIALS AND METHODS
All chemicals were purchased from Sigma Chemical Company (St. Louis, MO, United States) unless otherwise stated. Experimental procedures were approved and conducted under the requirements of the Animal Husbandry and Veterinary Research Institute of Tianjin, China.
2.1 Oocyte collection and IVM
Porcine ovaries were collected from a local slaughterhouse and transported to the laboratory in 0.9% saline at 25–30°C within 2 h after collection. COCs were aspirated from the follicles (2–8 mm in diameter) using a disposable 10 ml syringe with an 18-gauge needle. The IVM for COCs was performed as described previously (Yang et al., 2021). One hundred and twenty COCs were placed in 35-mm Petri dishes for each group with three repeats and cultured in 100 μl of the maturation medium under 250 μl mineral oil, 5% CO2 in air with 100% humidity at 38.5°C for 42 h.
2.2 Assessment of cumulus expansion index, oocyte survival rate and polar body rate
Cumulus expansion index was assessed as described previously (Prochazka et al., 2004). After IVM culture for 42 h, COCs from the three groups were examined, and the formula (sum of total COCs score/total number of COCs) was used to evaluate the cumulus expansion index. Oocyte survival rate was the number of survival oocytes/total number of the oocytes × 100%. The survival oocytes have intact zona pellucida and plasma membrane and clear perivitelline space without cytoplasmic leakage or oocyte shrinkage. The oocyte polar body was stained using Hoechst 33342 to evaluate polar body rate as described previously (Li et al., 2015), and the polar body rate was the percentage of oocytes with first polar body/total number of the oocytes × 100%.
2.3 Parthenogenetic development of embryos
The MII oocytes were washed thrice with activation medium and then exposed to one DC electrical pulse for activation after equilibrated in activation medium for 30–60 s. The activation medium contained 0.3 M mannitol, 0.05 mM CaCl2, 0.1 mM MgCl2, and 0.1% bovine serum albumin. The PA embryos were placed in 35-mm Petri dishes and cultured in porcine zygote medium 3 (Nánássy et al., 2008) under an incubator with 5% CO2 at 38.5°C.
2.4 Measurement of ROS level
ROS levels of oocytes were measured as described previously (Yang et al., 2021). Briefly, the matured oocytes from the three groups (n = 50, r = 3 for each group) were removed the cumulus cells and zona pellucida, washed three times in phosphate buffered saline (PBS), and then incubated with ROS working solution (DCFH-DA, 10 mM) for 20 min at 37°C. After washed three times in PBS, a fluorescence microscope (Olympus, BX60, Japan) with a digital camera (Nikon 990, Tokyo, Japan) was used to check the oocytes and capture the images of all oocytes. The ImageJ by Wayne Rasband from National Institute of Health (Bethesda, MD, United States) was used to analyze the images.
2.5 Mitochondrial distribution analysis
Mitochondrial distribution analysis (n = 10, r = 3 for each group) was performed as described previously (Cui et al., 2009). Briefly, after 42 h of IVM, the mitochondria of the oocytes were labeled by Mito-Tracker Red CMXRos. The fluorescence microscope (Olympus) with a digital camera (Nikon 990) was used to check the labeled oocytes and capture the images of all oocytes. There were two main distribution features of the mitochondrial distribution pattern for porcine oocytes, distributed evenly throughout the ooplasm, and distributed unevenly within the ooplasm as described previously (Cui et al., 2009). The value of mitochondrial distribution was analyzed in a blind manner and determined as the number of oocytes with homogeneous mitochondria/total number of the oocytes × 100.
2.6 Staining of blastocysts
Blastocyst rates of the three groups (n = 5, r = 3 for each group) were checked using bisbenzimide (Hoechst 33342) as described previously (Wan et al., 2009). The blastocysts were examined under the fluorescence microscope (Olympus) with a digital camera (Nikon 990). Blastocyst nuclei labeled with Hoechst 33342 appeared blue, and total cell numbers were counted.
2.7 Quantitative real-time PCR
Total RNA from the oocytes of the three groups (n = 50, r = 3 for each group) was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer's instructions, and genomic DNA was removed using DNase-I (GeneCopoeia, Rockville, MD, United States). In addition, cumulus cells from the COCs of the three groups (n = 100, r = 3 for each group) were peeled by gentle pipetting in 0.1% hyaluronidase after IVM, and total RNA from cumulus cells was also isolated as described above. Synthesis of cDNA was performed using a first strand cDNA synthesis kit (GeneCopoeia, Rockville, MD, United States) following the manufacturer's instructions, and a RT-qPCR Detection Kit (GeneCopoeia) was used for qPCR with 7900HT System (Applied Biosystems, Foster City, CA, United States). The primer sequences of GRP78, ATF4, CHOP, CASP3, BCL2, BAX, PTGS1, PTGS2, PTX3, and GAPDH genes were designed and synthesized by Shanghai Sangon Biotech Co., Ltd. (Table 1). The primers of GRP78, ATF4, CHOP, BAX, CASP3, BCL2, and GAPDH genes were used for the oocytes, while the primers of GRP78, ATF4, CHOP, BCL2, BAX, CASP3, PTGS1, PTGS2, PTX3, and GAPDH genes were used for the cumulus cells. The relative expression levels were calculated using 2-ΔΔCt analysis method (Livak & Schmittgen, 2001), and GAPDH was used as the endogenous control.
| Gene | Primer sequence | GenBank accession no. | Product size (bp) |
|---|---|---|---|
| GRP78 | F: ATATAAGCGGAGCAGGCGAC | XM_001927795.7 | 87 |
| R: GAGCTCTCACACACACGGAA | |||
| ATF4 | F: AGTCCTTTTCTGCGAGTGGG | NM_001123078.1 | 80 |
| R: CTGCTGCCTCTAATACGCCA | |||
| CHOP | F: TAATCCAGCCGCAGAGATGG | NM_001144845.1 | 96 |
| R: TCCTGCAGGTCCTCATACCA | |||
| CASP3 | F: TAACCCGAGTAAGAATGT | NM_214131.1 | 121 |
| R: ATACCAGTTGAGGCAGAC | |||
| BCL2 | F: CTTTGCCGAGATGTCCAGC | XM_021099593.1 | 138 |
| R: TCCACAGGGCGATGTTGTC | |||
| BAX | F: GCTTCAGGGTTTCATCCAGGA | XM_003127290 | 134 |
| R: CCAGTTCATCTCCAATGCGC | |||
| PTGS1 | F: CAACACGGCACACGACTACA | XM_001926129.6 | 157 |
| R: CTGCTTCTTCCCTTTGGTCC | |||
| PTGS2 | F: ACAGGGCCATGGGGTGGACT | NM_214321.1 | 121 |
| R: CCACGGCAAAGCGGAGGTGT | |||
| PTX3 | F: GGCCAGGGATGAATTTTAC | NM_001244783 | 185 |
| R: CTATCCTCTCCAACAAGTGA | |||
| GAPDH | F: TCAAATGGGGTGATGCTGGT | XM_021091114 | 124 |
| R: GCAGAAGGGGCAGAGATGAT |
2.8 Experiment design
In Experiment 1, COCs were treated with 0, 50, 100, and 150 μM TUDCA (Sigma, 35807-85-3) to determine the optimal concentration of TUDCA. The stock solution (1,000 μM TUDCA) was prepared using 0.0052 g TUDCA solved in 10 ml ultrapure water, and 50, 100, and 150 μM TUDCA was prepared using the stock solution. Cumulus expansion index, oocyte survival rate, and polar body rate were analyzed after IVM for 42 h. The presence of the first polar body was normally considered to be the oocyte maturity. After the non-treated MII oocytes were parthenogenetically activated, the parthenogenetic activation (PA) embryos were treated with 0, 50, 100, and 150 μM TUDCA. There were 80 PA embryos for each group with three repeats. Cleavage rates were calculated after cultured for 48 h, and the blastocyst rates were checked after cultured for 168 h.
In Experiment 2, COCs were treated with nothing, 50 μM BPA (BPA group) as described previously (Qi et al., 2014), and 50 μM BPA and 100 μM TUDCA (BPA + TUDCA group). Cumulus expansion index, oocyte survival rate, polar body rate, mitochondrial distribution, and ROS level were analyzed as described above. In addition, cumulus cells were peeled from the COCs after IVM. Expression of GRP78, ATF4, CHOP, CASP3, BCL2, and BAX in the oocytes and expression of GRP78, ATF4, CHOP, CASP3, BCL2, BAX, PTGS1, PTGS2, and PTX3 in the cumulus cells were analyzed. After the non-treated MII oocytes were parthenogenetically activated, PA embryos were treated with nothing, 50 μM BPA (BPA group), and 50 μM BPA and 100 μM TUDCA (BPA + TUDCA group). There were 80 PA embryos for each group with three repeats. Cleavage rate, blastocyst rate, and total cell number of blastocysts were checked as described above.
2.9 Statistical analysis
Data normality and homoscedasticity were tested using PROC UNIVARIATE procedure in SAS version 8 (SAS Institute Inc., Cary, NC, United States). One-way ANOVA followed by Duncan's test was used for equal variances, while Kruskal–Wallis one-factor ANOVA was performed to compare means with unequal variances. All data were expressed as mean ± standard deviation. A probability of P < 0.05 was considered statistically significant.
3 RESULTS
3.1 Effects of different concentrations of TUDCA on IVM and in vitro parthenogenetic development of embryos
The cumulus expansion indexes and polar body rates of COCs treated with 100 and 150 μM TUDCA were significantly higher than those of COCs treated with 0 and 50 μM TUDCA (P < 0.05; Figure 1a), but TUDCA treatment (50–150 μM) had no significant effect on the survival rates (P > 0.05). In addition, the cleavage rates and blastocyst rates of parthenogenetic development of embryos treated with 100 and 150 μM TUDCA were significantly higher than those of embryos treated with 0 and 50 μM TUDCA (P < 0.05; Figure 1b). Furthermore, the cleavage rates of parthenogenetic embryos treated with 100 μM TUDCA was lower comparing to that treated with 150 μM TUDCA (P > 0.05), but the blastocyst rate of parthenogenetic embryos treated with 100 μM was higher than that of parthenogenetic embryos treated with 150 μM TUDCA (P < 0.05; Figure 1b).
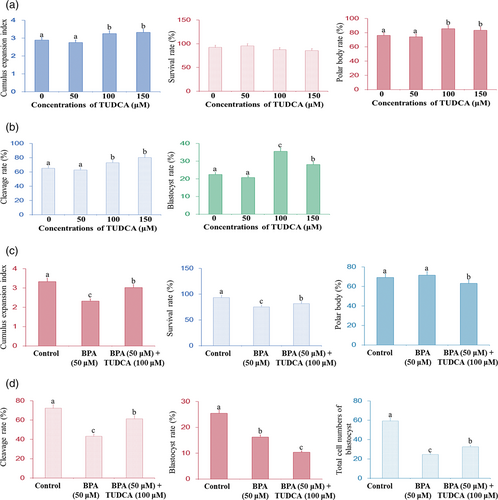
3.2 Effect of BPA and TUDCA on IVM and parthenogenetic development of embryos
Figure 1c shows that the cumulus expansion indexes and survival rate of COCs treated with BPA were significantly lower than control group (P < 0.05), but the cumulus expansion indexes and survival rate of COCs treated with BPA and TUDCA were significantly higher than that treated with BPA (P < 0.05). Nevertheless, BPA treatment had no effects on polar body rates of COCs (P > 0.05; Figure 1c), and TUDCA treatment had favorable effects on polar body rates of COCs (P < 0.05).
Figure 1d showed that BPA treatment decreased the cleavage rate, blastocyst rate, and total cell number of blastocyst of parthenogenetic embryos (P < 0.05) but BPA and TUDCA treatment increased the cleavage rate and total cell number of blastocyst of parthenogenetic embryos comparing with BPA treatment (P < 0.05). However, BPA and TUDCA treatment had adverse effect on the blastocyst rate (P < 0.05).
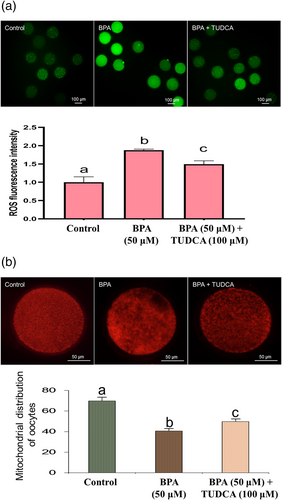
3.3 Effect of BPA and TUDCA on intracellular level of ROS and mitochondrial distribution in the porcine oocytes
It was shown in Figure 2a that the intracellular ROS level of the oocytes in control group was significantly lower than other two groups (P < 0.05), suggesting that BPA treatment increased the intracellular ROS level of the oocytes (P < 0.05). However, TUDCA treatment could inhibit the effect of BPA treatment on intracellular ROS level (P < 0.05). Figure 2b revealed that BPA treatment had adverse effect on mitochondrial distribution of the oocytes (P < 0.05), but TUDCA treatment could attenuate the adverse effect of BPA treatment on mitochondrial distribution of the oocytes (P < 0.05).
3.4 Effects of BPA and TUDCA on expression of ER stress and apoptosis related genes in the porcine oocytes
The RT-qPCR assay showed (Figure 3a) that BPA treatment significantly increased the mRNA levels of all ER stress related genes, including GRP78, ATF4, and CHOP, in the oocytes (P < 0.05). However, TUDCA treatment significantly suppressed expression of ATF4, GRP78, and CHOP mRNA induced by BPA (P < 0.05). Furthermore, BPA treatment also significantly enhanced mRNA expression of all apoptosis related genes, including BAX, BCL2, and CASP3 (P < 0.05), but TUDCA treatment significantly attenuated the effects on expression of BAX and CASP3 induced by BPA (P < 0.05). However, BPA + TUDCA treatment significantly upregulated the expression level of BCL2 comparing to the control and BPA groups (P < 0.05).
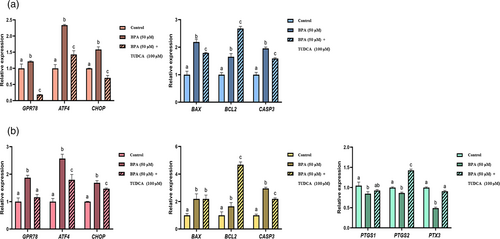
3.5 Effects of BPA and TUDCA on expression of ER stress, apoptosis, and cumulus expansion related genes in the cumulus cells
It was showed in Figure 3b that BPA treatment significantly increased the relative levels of GRP78, ATF4, and CHOP mRNA in the cumulus cells (P < 0.05), but TUDCA treatment significantly weakened the effects on expression of these ER stress related genes induced by BPA. BPA treatment also significantly improved expression of BAX, BCL2, and CASP3 (P < 0.05), but TUDCA treatment significantly inhibited the effect on expression of CASP3 induced by BPA (P < 0.05). However, BPA + TUDCA treatment significantly upregulated BCL2 level comparing to the control and BPA groups (P < 0.05), and there was no significant difference between BPA + TUDCA group and BPA group in the BAX expression level (P > 0.05).
BPA treatment significantly suppressed expression of all cumulus expansion related gene mRNA, including PTGS1, PTGS2, and PTX3 (P < 0.05), but TUDCA treatment significantly weakened the effect on expression of PTX3 induced by BPA (P < 0.05). However, there was no significant difference between BPA + TUDCA group and BPA group in the PTGS1 expression level (P > 0.05), but BPA + TUDCA treatment enhanced the expression level of PTGS2 comparing to the control and BPA groups (P < 0.05).
4 DISCUSSION
Our results indicated that treatment with 100 μM TUDCA had beneficial effects on the cumulus expansion indexes and polar body rates of COCs. During oocyte maturation in mammals, some adverse factors induce activation of ER stress, which has negative effects on the oocyte maturation and pre-implantation embryo development (Lin et al., 2019). As a selective inhibitor of ER stress, TUDCA (100 μM) can significantly improve the maturation rate during IVM in the bovine (Khatun, Ihara, et al., 2020), and the maturation rates of oocytes treatment with 50 μM of TUDCA are significantly enhanced during porcine oocyte IVM (Zhang et al., 2012). Therefore, the 100 μM of TUDCA may be the best concentration for porcine oocyte IVM.
In this study, treatment with 100 or 150 μM TUDCA had favorable effects on the cleavage rates and blastocyst rates of parthenogenetic embryos. TUDCA treatment (100 μM) during parthenogenetic activation can decrease ER stress, which improves in vitro development of parthenogenetic embryos in pigs (Park, Kim, et al., 2018). After parthenogenetic activation, intra-oocyte injection of TUDCA (a final concentration of 50 μM) significantly decreases early embryo development. However, media-supplemented with 50 μM TUDCA improves early PA embryo development but has no significant effects on cleavage rates in pigs (Dicks et al., 2021). Therefore, treatment with 100 μM TUDCA is the best concentration for improving the blastocyst quality in the porcine.
Our data showed that treatment with BPA had adverse effects on the cumulus expansion indexes and survival rates of COCs, as well as the cleavage rate, blastocyst rate, and total cell number of blastocyst of parthenogenetic embryos. BPA impairs oocyte meiotic division and reduces the expansion of cumulus cells and bidirectional communication in the COCs of mice (Acuña-Hernández et al., 2018). BPA exposure impairs the survival and decreases pairing-synapsis and meiotic recombination of in vitro oocytes in humans (Brieño-Enríquez et al., 2011). It has been reported that treatment with 25 μM BPA induces oxidative stress damages of follicles and suppresses in vitro follicular development and oocyte maturation in mice. Therefore, treatment with BPA had unfavorable effects on the IVM, and in vitro development of parthenogenetic embryos, but TUDCA treatment could attenuate the negative effects induced by BPA except polar body rate and blastocyst rate.
It was found in this study that BPA treatment enhanced the intracellular ROS level of the oocytes but TUDCA treatment weakened the effect induced by BPA. Treatment with 0.05 mg/ml of BPA elevates ROS level and changes enzyme expression in the bovine oocytes (Nguyen et al., 2022). Addition of TUDCA in oocyte maturation medium ameliorates the adverse effects induced by tunicamycin and significantly decreases the ROS level in the bovine COCs (Khatun, Ihara, et al., 2020). TUDCA supplementation markedly reduces ROS level but increases blastocyst developmental rate, trophectoderm proportion, and cell survival of bovine early embryos (Yoon et al., 2014). Therefore, the unfavorable effects of BPA and beneficial impacts of TUDCA on IVM may be related to intracellular ROS level in the porcine oocytes.
This study indicated that BPA treatment decreased mitochondrial distribution of the oocytes but TUDCA supplementation attenuates the effect induced by BPA. BPA exposure disturbs the quality of mature oocytes, which accompanies with abnormal distribution and decreased mitochondrial membrane potential in mice (Pan et al., 2021). BPA exposure leads to upregulation of mitochondria-related antioxidant enzymes during porcine IVM (Park, Park, et al., 2018). Δ9-tetrahydrocannabinol diminishes mitochondrial respiration and decreases abundance of mitochondrial chain complex proteins, but TUDCA can abrogate these negative effects induced by Δ9-tetrahydrocannabinol in the human BeWo trophoblasts (Lojpur et al., 2019). Therefore, the unfavorable effects of BPA on mitochondrial distribution of the oocytes could be inhibited by TUDCA during IVM in the porcine oocytes.
Results revealed that BPA treatment enhanced expression of GRP78, ATF4, and CHOP mRNA but TUDCA supplementation weakened these effects induced by BPA in the oocytes and cumulus cells. BPA exposure increases expression of GRP78 accompanied with ER stress (Pan et al., 2021) and induces upregulation of ATF4 and CHOP in immortalized murine hypothalamic cell lines (Xu et al., 2022). BPA induces ER stress, which results in the upregulation of CHOP and GRP78 genes in mouse non-parenchymal hepatocytes (Asahi et al., 2010). However, addition of TUDCA downregulates GRP78, ATF4, and CHOP genes in the bovine COCs and in vitro derived embryos (Khatun, Ihara, et al., 2020; Khatun, Wada, et al., 2020); decreases of ER stress; and attenuates palmitate-induced expression of GRP78, CHOP, and ATF4 in rat islet β-cells (Zhu et al., 2013). Therefore, TUDCA could reduce ER stress induced by BPA, which partly was via modulating expression of GRP78, CHOP, and ATF4 genes in the porcine oocytes and cumulus cells.
This study demonstrated that BPA exposure upregulated expression of BAX, BCL2, and CASP3 in the porcine oocytes and cumulus cells but TUDCA treatment weakened these effects on expression of BAX and CASP3 in the porcine oocytes and CASP3 in cumulus cells induced by BPA. However, BPA + TUDCA treatment increased the expression of BCL2 in the porcine oocytes and cumulus cells. BPA enhances BAX and CASP3 expression levels and apoptosis rate in the mouse monocyte cell line RAW 264.7 (Wu et al., 2022), promotes apoptosis, and increases expression of anti-apoptotic protein (BCL2) in human choriocarcinoma BeWo cells (Ponniah et al., 2015). However, addition of TUDCA in culture medium downregulates expression of pro-apoptotic (BAX) gene but upregulates BCL2 expression in the bovine COCs and in vitro derived embryos (Khatun, Ihara, et al., 2020; Khatun, Wada, et al., 2020). Supplementation of TUDCA reduces expression of BAX but increases the transcription of BCL2 in the porcine embryos (Li et al., 2017). TUDCA treatment decreases CASP3 expression, which is favorable for in vitro development of somatic cell nuclear transfer embryos in the porcine (Park et al., 2019). Therefore, TUDCA may improve in vitro oocyte maturation and embryonic development in pigs, mainly through downregulation of the CASP3 and BAX genes, but not BCL2. In addition, overexpression of BCL2 may have adverse effect on the blastocysts rate in the porcine.
Our results found that BPA treatment inhibited expression of PTGS1, PTGS2, and PTX3 but TUDCA supplementation weakened the effects on expression of PTGS1 and PTX3 induced by BPA and improved PTGS2 expression. Treatment with gonadotrophin improves oocyte developmental quality and also increases expression of PTGS1 in human ovary (Adriaenssens et al., 2010). Expression levels of cumulus expansion-related genes decline in the cumulus cells from endometriosis-related infertile women compared to the controls (Yin, Mao, et al., 2021). During cumulus expansion, cumulus cells produce PTX3 that is necessary for female fertility (Salustri et al., 2004). Tannins supplementation in the maturation medium improves oocyte cytoplasmic maturation and subsequent embryonic development and also enhances the cumulus expansion index, expression of cumulus-expansion-related genes (PTGS1, PTGS2, and PTX3) in pigs (Yin, Sun, et al., 2021). Therefore, BPA suppressed cumulus expansion, and TUDCA could improve cumulus expansion, which may be related to the expression of PTGS2 and PTX3 in the porcine cumulus cells.
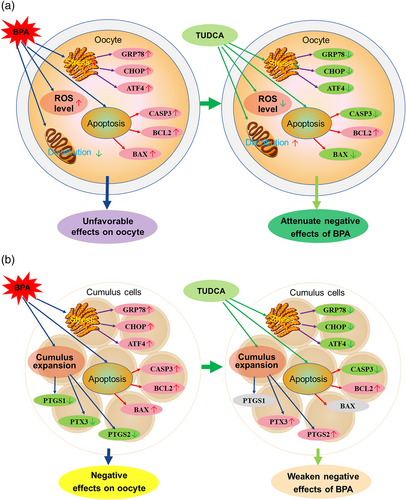
In conclusion, BPA exposure decreased the cumulus expansion, survival rate, polar body rate, mitochondrial distribution of oocytes, and the cleavage rate and blastocyst rate of the parthenogenetic embryos but upregulated expression of GRP78, ATF4, CHOP, CASP3, BCL2, and BAX in the oocytes and cumulus cells. However, BPA treatment downregulated expression of PTGS1, PTGS2, and PTX3 genes in the cumulus cells. In addition, TUDCA treatment could relieve these adverse effects induced by BPA except polar body rate, blastocyst rate, and expression of BCL2 and PTGS1 (Figure 4a,b). Therefore, BPA exposure had unfavorable effects on IVM, and in vitro development of parthenogenetic embryos, but TUDCA treatment could partly attenuate these negative effects induced by BPA, which may be associated with the genes related to ER stress, apoptosis, and cumulus expansion, as well as intracellular ROS level and mitochondrial distribution of oocytes.
ACKNOWLEDGMENTS
This work was supported by the Major Special Project of Seed Industry of Tianjin Municipal Bureau of Science and Technology, China (19ZXZYSN00110), and the Hebei Natural Science Foundation, China (C2022402038).
CONFLICT OF INTEREST
The authors declare no personal conflict of interest.




