Considering potential roles of selected MicroRNAs in evaluating subclinical mastitis and Milk quality in California mastitis test (+) and infected bovine milk
Abstract
This study investigates the relationships between subclinical mastitis and milk quality with selected microRNAs in cow milk. California Mastitis Test (CMT)-positive (n = 20) and negative (n = 20) samples were compared (Experiment I). Additionally, samples with CMT-positive but microbiological-negative, as well as positive for only Staphylococcus subspecies (Staph spp.) and only Streptococcus subspecies (Strep spp.) were examined (Experiment II). Four groups were formed in Experiment II: Group I (CMT and microbiological-negative) (n = 20), Group II (CMT-positive but microbiological-negative) (n = 10), Group III (Staph spp.) (n = 5), Group IV (Strep spp.) (n = 5). While electrical conductivity, somatic cell count (SCC), malondialdehyde (MDA) increased, miR-27a-3p and miR-223 upregulated and miR-125b downregulated in the CMT-positive group in Experiment I. SCC and MDA were higher in CMT-positive groups. miR-27a-3p and miR-223 upregulated in Groups III and IV. While miR-155 is upregulated, miR-125b downregulated in Group IV. Milk fat is positively correlated with miR-148a and miR-223. As miR-27a-3p positively correlated with SCC and MDA, miR-125b negatively correlated with electrical conductivity and SCC. miR-148a and MDA were positively correlated. miR-155 was correlated with fat-free dry matter, protein, lactose, and freezing point. miR-223 was positively correlated with SCC and miR-148a. Results particularly highlight miR-27a-3p and miR-223 as potential biomarkers in subclinical mastitis, especially those caused by Staph spp. and Strep spp., while miR-148a, miR-155, and miR-223 stand out in determining milk quality.
1 INTRODUCTION
Milk is a biological fluid that begins with colostrum following birth in mammals. It is an essential component for the health and development of offspring and is regarded as a high-quality food due to ingredients such as vitamins, minerals, and essential amino acids, as well as its fat and carbohydrate content (Claeys et al., 2013; Khastayeva et al., 2021). In addition to consumption requirements, milk production goals are increasing based on growing awareness of nutrition. Quality in animal products is directly related to the quantity and proportions of substances found in the animal product, as well as processes such as product preservation and processing (Nickerson, 2006; Yakan et al., 2021). In this context, key parameters considered in the evaluation of milk quality include somatic cell count (SCC), lactose, protein, fat, and fat-free dry matter (FFDM).
The formation of milk quality is influenced by animal health besides genetics, ration, lactation period, and management (El-Sayed & Kamel, 2021; Shaheen et al., 2020). In dairy cattle husbandry, mastitis, an inflammation of the mammary gland, is a significant disease that leads to crucial economic problems and impairs milk quality. Mastitis is a global issue in dairy cattle husbandry, and it creates a constant risk of infection within and between herds due to various environmental and microbial predisposing factors (Abed et al., 2021; Thomas et al., 2018). In addition, toxins present in milk obtained from sick animals and disruptions in milk composition not only result in quality loss but also have adverse effects on public health.
Mastitis is basically categorized into clinical and subclinical mastitis. While diagnosis of clinical mastitis relatively easy, subclinical mastitis is difficult to diagnose because it does not exhibit clinical symptoms. Subclinical mastitis, which is an asymptomatic disease, is characterized by increased somatic cell count (SCC), altered milk composition, and low milk yield (Martins et al., 2020). It is reported that the incidence of subclinical mastitis in herds can reach up to 50% (Gangani et al., 2022). Even if SCC and California Mastitis Test (CMT) are widely used for determination of mammary health, these parameters are not sufficient for subclinical mastitis diagnosis due to affecting factors such as lactation period, milking frequency, and timing (Tzelos et al., 2022).
Over 90% of mastitis cases are attributed to bacterial infections, constituting the predominant cause among all mastitis diagnoses (Gonçalves et al., 2018). Staphylococcus subspecies (Staph spp.) and Streptococcus subspecies (Strep spp.) are among the most common pathogens associated with subclinical mastitis development in cattle. Contagious mastitis caused by Staph spp. is characterized by a significant increase in SCC (Martins et al., 2020). The success of lactation antibiotic treatment for subclinical mastitis diagnosed with Staph spp. is limited, and the disease in animals can become chronic (Khasapane et al., 2023; Sağlam et al., 2017). While Strep spp. do not lead to a substantial increase in SCC, infected animals show resistance to antibiotic treatment, and the disease tends to persist over prolonged periods (Bal et al., 2010; Keyvan, 2023).
The availability of microRNAs (miRNAs) as biomarkers has been assessed for many disorders in recent years. miRNAs are small, single-stranded, non-coding RNA molecules approximately 19–25 nucleotides in length. These molecules are highly conserved across species and play a key role in regulating gene expression in various cells in the organism (Cai et al., 2018). Research has shown that miRNAs are involved in the regulation of more than 60% of protein synthesis in mammals and are involved in molecular pathways related to cell proliferation, the immune system, and inflammation (Quan et al., 2020).
While there have been numerous studies identifying multiple miRNAs and investigating their activities in studies assessing physiological and pathological conditions, research on miRNAs related to subclinical mastitis and milk quality in cattle is limited (Sun et al., 2019; Tzelos et al., 2022). In this study, the activity of five miRNAs, which are thought to play a role in pathways such as inflammation, oxidative stress, and apoptosis, and whose regulation may vary in subclinical mastitis, has been investigated (Feng et al., 2021; Luo et al., 2020; Valmiki et al., 2020). The selection of miRNAs took into consideration not only the relevant pathways but also potential impacts on milk composition, such as carbohydrate and fat metabolism, due to their possible effects on milk quality (Cai et al., 2021; Duman et al., 2022; Fang et al., 2016; Quan et al., 2020). Therefore, we aimed to explore potential relationships between subclinical mastitis and milk quality parameters with miR-27a-3p, miR-125b, miR-148a, miR-155, and miR-223 which are scarcely studied in the milk of Holstein cows during lactation. In the study, CMT-positive samples were compared with CMT-negative samples. Additionally, samples with positive CMT results but negative microbiological analysis results, as well as samples positive for only Staph spp. and only Strep spp. based on microbiological analysis, were also examined. The relevant miRNAs are suggested to play a role in the regulation of numerous pathways at the molecular level, particularly in inflammation (Luo et al., 2020; Luoreng et al., 2021; Valmiki et al., 2020).
2 MATERIALS AND METHODS
2.1 Sample collection, measuring parameters, microbiological analysis, and design of the study
The study used milk samples obtained from 40 clinically healthy multiparous Holstein cows (lactation parities: 2.43 ± 0.08) aged 3–4 years during mid-lactation from a private farm in Hatay, Turkiye. The samples were collected during the morning milking. Before collecting the milk samples, the udders of animals were immersed in teat-dipping solutions and cleaned with 70% ethyl alcohol. The initial milk obtained was discarded and samples were collected aseptically. Following CMT application, 150 ml of milk samples were collected in triplicate into 50 ml sterile, nuclease-free falcon tubes.
The collected samples were quickly transported to the laboratory in the cold chain. A total of 50 ml of the samples was stored at −80°C. Another 50 ml volume of the samples was centrifuged at 1800 xg for 15 min at +4°C to remove the cream layers. After removing the cream layers with a sterile spatula, the skim milk layers were transferred to nuclease-free tubes with a volume of 15 ml and stored −80°C for molecular analysis.
While 10 ml of milk was used for microbiological analysis, SCC was determined using a Somatic Cell Count Device (Lactoscan SCC 6010, Bulgaria). In addition to pH values (Hanna pH meter, HI83141, USA), major milk quality parameters (fat, fat-free dry matter (FFDM), protein, lactose, freezing point, and electrical conductivity) were determined using a Milkotester Master Classic device (M2, P1, Bulgaria) from a total of 35 ml of milk (Özkan et al., 2020). In addition, MDA levels of the samples were determined with a UV-spectrophometer at 532 nm wavelength (Esterbauer & Cheeseman, 1990).
Following homogenization, 20 μl of milk samples were spread onto 5% Sheep Blood Agar (Sigma, USA) and MacConkey Agar (Merck, Germany). The culture plates were incubated at 37°C for 24–48 hours, and colonies were purified through subcultures. Pure colonies were evaluated for hemolysis, Gram staining, catalase, and oxidase test characteristics (Oliver et al., 2004). Subcultured pure colonies were analyzed using MALDI-TOF MS (Bruker Daltonics GmbH, Germany) with the ethanol formic acid extraction method (Theel et al., 2012). The spectra obtained with the instrument's Flex Control software program (Biotyper 3.0; Microflex LT; Bruker Daltonics GmbH, Bremen, Germany) were compared using the MALDI Biotyper Real-Time Classification (RTC) software to identify the colonies. Results scoring between 1,700 and 3,000 were considered correct.
Based on the CMT and microbiological analysis, two experiments were designed. In Experiment I, according to the CMT results two groups were constructed as CMT (−) and CMT (+) groups. On the other hand, samples were divided into four groups in Experiment II: Group I was the Control Group which had both CMT and microbiological analysis results negative samples. Samples in Group II were CMT-positive but microbiologically negative. In addition, in Group III both CMT and microbiological analysis results were positive. While samples in Group III had Staphylococcus spp., Group IV samples had Streptococcus spp. Detailed information about experiments are presented in Table 1.
| Experiments | Groups | CMT | Microbiological analysis |
|---|---|---|---|
| Experiment I | CMT (−) group (n = 20) | Negative | Negative |
| CMT (+) group (n = 20) | Positive | Both negative and positive | |
| Experiment II | Group I (Con) (n = 20) | Negative | Negative |
| Group II (n = 10) | Positive | Negative | |
| Group III (n = 5) | Positive | Positive (Staph spp.) | |
| Group IV (n = 5) | Positive | Positive (Strep spp.) |
- Con: Control; Staph spp: Staphylococcus spp.; Strep spp: Streptococcus spp.
2.2 RNA isolation, cDNA synthesis, and qPCR application
Total RNA isolation from skim milk samples was performed using a modified Trizol method as described by Rio et al. (2010). After thawing at +4°C and vortexing, 250 μl of skim milk samples stored -80°C was homogenized with 750 μl of Trizol Reagent (Cat. No: 15596018, ThermoFisher Scientific, USA). Following chloroform, isopropyl alcohol, and ethyl alcohol steps, obtained RNA samples were left to air-dry at room temperature for approximately 10 min and were then dissolved in 15 μl of nuclease-free water. The purity and concentration values were determined using a nucleic acid spectrophotometer (Merinton-SMA 1000, China).
The miRNAs in the samples were polyadenylated using a thermal cycler (Biorad T100, USA) with incubation at 37°C for 30 min, following the Poly(A) Polymerase protocol (Cat. No: E017, ABM, CA, USA). Subsequently, cDNA synthesis was conducted using the OneScript Plus cDNA synthesis kit (Catalog No: G236, ABM, CA, USA). According to the kit protocol, 10 μl of the reaction products from the Poly(A) Polymerase kit were taken, and a final volume of 20 μl was obtained by adding 4 μl of 5X RT Buffer, 1 μl of dNTP (10 mM), 1 μl of oligo (dT) primer (10 μM), 1 μl of OneScript® RTase (200 U/μL), 0.5 μl of RNaseOFF Ribonuclease Inhibitor (40 U/μL), and nuclease-free water. After vortexing and centrifugation of the samples, cDNA synthesis was carried out by incubating at 50°C for 15 min and 85°C for 5 min. The cDNA samples were diluted 10-folds and stored at −20°C until qPCR applications.
Amplifications of miR-27a-3p, miR-125b, miR-148a, miR-155, and miR-223, and the U6 (housekeeping) were carried out using qPCR (Rotor Gene Q MDx 5PLEX HRM, Qiagen, USA). For this purpose, the qPCR protocol was set as follows: Denaturation at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 sec, annealing at 60°C (arranged according to primers properties) for 60 sec, and extension at 72°C for 30 sec (Table 2). The forward sequences for amplifying these miRNAs were designed using the miRbase database (https://mirbase.org/), and the Universal 3’ Reverse Primer (Cat. No: MPH00000, ABM, CA, USA) was used as the reverse primer.
| miRNAs | Forward primer sequences | Annealing temperatures |
|---|---|---|
| Bta-miR-27a-3p | 5’-TTCACAGTGGCTAAGTTCCG-3′ | 63.00°C |
| Bta-miR-125b | 5’-TCCCTGAGACCCTAACTTGTG-3′ | 57.00°C |
| Bta-miR-148a | 5’-TCAGTGCACTACAGAACTTTGT-3′ | 55.20°C |
| Bta-miR-155 | 5’-TTAATGCTAATCGTGATAGGGGT-3′ | 64.50°C |
| Bta-miR-223 | 5’-TGTCAGTTTGTCAAATACCCCA-3′ | 61.00°C |
| U6 | 5’-GCAGGGGCCATGCTAATCTTCTCTGTATCG-3′ | 61.00°C |
2.3 Statistical analysis
The data were evaluated firstly in terms of parametric test assumptions such as normality and homogeneity of variances. In Experiment I, Student's t-test was used to examine the differences in milk quality parameters between CMT (−) and CMT (+) groups, while the Mann–Whitney U test was used to evaluate SCC and MDA parameters. In Experiment II, the differences in milk quality parameters between groups were evaluated with a one-way analysis of variance (ANOVA) and the Duncan test as a post-hoc test. Also, SCC and MDA were evaluated with the Kruskal-Wallis test with multiple Dunn tests. In addition, the Spearman correlation coefficient was performed to assess the correlation between SCC, MDA, milk quality parameters, and miRNA expression levels. Expression levels of miRNAs were calculated by the 2−ΔΔCt method (Livak & Schmittgen, 2001). Receiver operating characteristic (ROC) analysis was used to determine the potential biomarker of miRNA expression levels. Sensitivity, specificity, area under the curve (AUC), and the cut-off values were calculated for each miRNA. Differences with P < 0.05 were considered statistically significant. Results were calculated as “Mean ± Standard Error of Mean” and presented as tables and figures. Statistical analyses were performed using Stata SE version 15.1.
3 RESULTS
According to the results, 20 samples were CMT positive while another 20 samples were CMT negative. In Experiment I, most of the measured parameters were similar except for electrical conductivity, SCC, and MDA. Compared to CMT (−) group, electrical conductivity and SCC levels were increased in CMT (+) group (P < 0.05 and P < 0.001, respectively). In addition, MDA levels were almost 40% higher in this group (P < 0.001) (Table 3).
| Parameters | CMT (−) | CMT (+) | P |
|---|---|---|---|
| Fat (%) | 3.16 ± 0.22 | 3.11 ± 0.19 | 0.884* |
| FFDM (%) | 9.59 ± 0.06 | 9.61 ± 0.13 | 0.890* |
| Protein (%) | 3.48 ± 0.02 | 3.50 ± 0.05 | 0.701* |
| Lactose (%) | 5.24 ± 0.03 | 5.26 ± 0.07 | 0.851* |
| Freezing point (°C) | −0.61 ± 0.01 | −0.62 ± 0.01 | 0.618* |
| Electrical conductivity (μS) | 4.80 ± 0.01 | 4.91 ± 0.05 | 0.042* |
| pH | 6.68 ± 0.01 | 6.65 ± 0.02 | 0.250* |
| SCC (×1000) | 86.00 ± 12.96 | 594.20 ± 224.29 | <0.001# |
| MDA (nmol/mL) | 17.75 ± 1.55 | 23.44 ± 0.66 | <0.001# |
- * : Student T test, #: Mann–Whitney U test, FFDM: Fat-Free Dry Matter, SCC: Somatic Cell Count, MDA: Malondialdehyde.
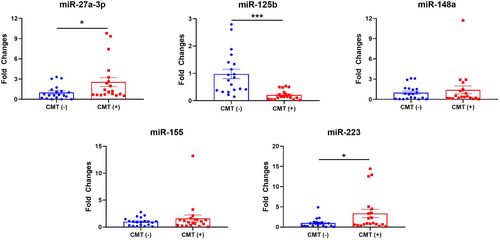
Compared to CMT (−) group, miR-27a-3p was found approximately 3-fold higher in CMT (+) group. Moreover, miR-125b was found almost 5-fold lower in CMT (+) group (P < 0.001). Expression levels of miR-223 were also higher more than 3-fold in CMT (+) group, compared to CMT (−) (P < 0.05) (Figure 1).
Based on the MALDI-TOF MS results, among the samples in Group III, Staphylococcus aureus was detected in three samples, while Staphylococcus chromogenes was identified in two samples. In addition, in Group IV, three samples were found to contain Streptococcus uberis, while Streptococcus agalactiae was present in two samples in Experiment II. In this experiment, most of the measured quality parameters were similar between groups. On the other hand, SCC was significantly lower in Group I (Control) than other groups. Even if the SCC was shown similar in other groups, the highest SCC was found in Group III (P < 0.001). MDA levels were found higher in all CMT-positive groups (Groups II, III, and IV), but the differences were significant only in Groups II and III (P < 0.01). Results are presented in Table 4.
| Parameters | Group I | Group II | Group III | Group IV | P |
|---|---|---|---|---|---|
| Fat (%) | 3.16 ± 0.22 | 3.36 ± 0.28 | 2.90 ± 0.34 | 2.80 ± 0.42 | 0.6691 |
| FFDM (%) | 9.59 ± 0.06 | 9.84 ± 0.07 | 9.30 ± 0.41 | 9.46 ± 0.26 | 0.1301 |
| Protein (%) | 3.48 ± 0.02 | 3.58 ± 0.03 | 3.38 ± 0.15 | 3.46 ± 0.09 | 0.1251 |
| Lactose (%) | 5.24 ± 0.03 | 5.38 ± 0.05 | 5.10 ± 0.23 | 5.16 ± 0.13 | 0.1491 |
| Freezing point (°C) | −0.61 ± 0.01 | −0.63 ± 0.01 | −0.61 ± 0.01 | −0.60 ± 0.02 | 0.4421 |
| Electrical conductivity (μS) | 4.80 ± 0.01 | 4.95 ± 0.10 | 4.86 ± 0.05 | 4.86 ± 0.02 | 0.1401 |
| pH | 6.68 ± 0.01 | 6.62 ± 0.03 | 6.72 ± 0.06 | 6.65 ± 0.03 | 0.0771 |
| SCC (×1000) | 86.00 ± 12.96b | 269.80 ± 48.95a | 1428.00 ± 840.45a | 409.20 ± 61.12a | <0.001 2 |
| MDA (nmol/mL) | 17.75 ± 1.55b | 24.07 ± 0.92a | 23.09 ± 0.45a | 22.53 ± 2.00ab | 0.004 2 |
- 1 : One-way ANOVA, 2: Kruskal-Wallis Test, a, b: Lowercases in the same line represent the difference between groups (P < 0.05), FFDM: Fat-Free Dry Matter, SCC: Somatic Cell Count, MDA: Malondialdehyde, Group I: Both CMT and microbiological results negative, Group II: CMT positive and microbiological results negative, Group III: CMT and Staph spp. positive, Group IV: CMT and Strep spp. positive.
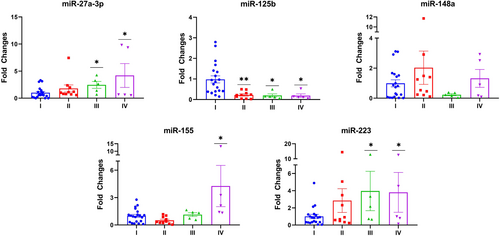
In addition to quality parameters, expression levels of most of the studied miRNAs were increased. Compared to Group I (Control), miR-27a-3p and miR-223 levels were significantly upregulated in Groups III and IV (P < 0.05). While miR-155 was upregulated almost 4-fold in Group IV, miR-125b levels were downregulated in all groups compared to Group I. Insignificant changes were also detected in terms of miR-148a levels in groups (Figure 2).
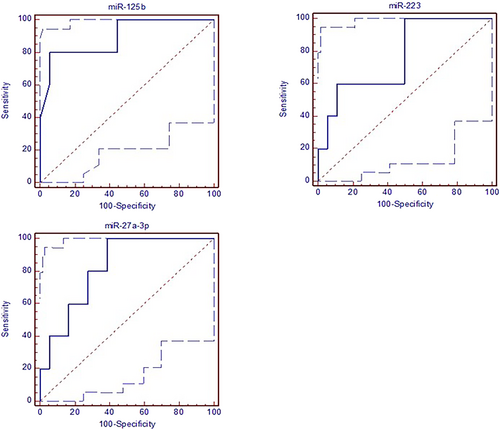
ROC curves and the area under the curve (AUC) values through Group I – Group III and Group I – Group IV were presented in Figures 3 and 4. The cut-off values of miR-27a-3p, miR-125b, and miR-223 were >0.65, ≤0.16, and >0.46, respectively Group I – Group III. AUC values for miR-27a-3p, miR-125b, and miR-223 were determined as 0.822 (p < 0.01), 0.894 (p < 0.001), and 0.767 (p < 0.05), respectively in Group I – Group III. Additionally, the sensitivity and specificity of miR-27a-3p were 100 and 61.1%, miR-125b were 80 and 94.4%, and miR-223 were 100 and 50% (Figure 3).
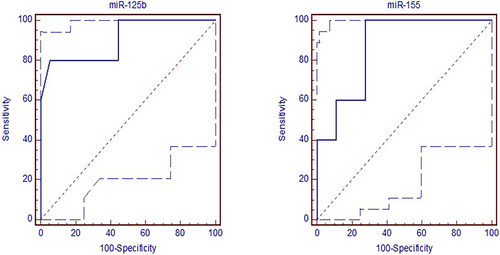
In Group I – Group IV, the cut-off values of miR-125b and miR-155 were ≤0.15 and >1.14, respectively. AUC values for miR-125b and miR-155 were determined as 0.906 (p < 0.001) and 0.867 (p < 0.001), respectively in Group I – Group IV. Moreover, the sensitivity and specificity of miR-125b were 80% and 94.4%, and of miR-155 were 100% and 72.2% (Figure 4).
Correlations between the studied parameters and miRNAs were also evaluated. Milk fat was found positively correlated with miR-148a (r: 0.445; P < 0.01) and miR-223 (r: 0.335; P < 0.05). While miR-27a-3p was positively correlated with SCC (r: 0.348; P < 0.05) and MDA (r: 0.424; P < 0.01), a negative correlation was found between miR-125b and electrical conductivity (r: -0.428; P < 0.01) and SCC (r: -0.368; P < 0.05). A positive correlation was also found between miR-148a and MDA (r: 0.345; P < 0.05). In addition, miR-155 was correlated with FFDM (r: -0.379; P < 0.05), protein (r: -0.345; P < 0.05), lactose (r: 0.416; P < 0.01), and freezing point (r: 0.360; P < 0.05). Furthermore, miR-223 was positively correlated with SCC (r: 0.324; P < 0.05) and miR-148a (r: 0.560; P < 0.001) (Table 5).
| Parameters | miR-27a-3p | miR-125b | miR-148a | miR-155 | miR-223 |
|---|---|---|---|---|---|
| Fat | 0.153 | 0.220 | 0.445** | −0.185 | 0.335* |
| FFDM | 0.026 | 0.076 | 0.263 | −0.379* | 0.109 |
| Protein | 0.058 | 0.050 | 0.228 | −0.345* | 0.126 |
| Lactose | 0.029 | 0.088 | 0.221 | −0.416** | 0.080 |
| Freezing point | −0.066 | −0.168 | −0.303 | 0.360* | −0.164 |
| Electrical conductivity | 0.196 | −0.428** | 0.080 | 0.132 | 0.248 |
| pH | −0.133 | 0.076 | −0.282 | 0.187 | −0.200 |
| SCC | 0.348* | −0.368* | 0.121 | 0.118 | 0.324* |
| MDA | 0.424** | −0.241 | 0.345* | −0.176 | 0.286 |
| miR-27a-3p | 1 | −0.215 | 0.275 | 0.222 | 0.241 |
| miR-125b | 1 | −0.031 | −0.160 | −0.151 | |
| miR-148a | 1 | −0.018 | 0.560*** | ||
| miR-155 | 1 | 0.060 | |||
| miR-223 | 1 |
- *: Correlation is significant at the 0.05 level (2-tailed), **: Correlation is significant at the 0.01 level (2-tailed), ***: Correlation is significant at the 0.001 level (2-tailed), FFDM: Fat-Free Dry Matter, SCC: Somatic Cell Count, MDA: Malondialdehyde.
4 DISCUSSION
Subclinical mastitis in cows is a chronic and costly problem characterized by loss of quality for the dairy sector. While CMT and SCC are commonly used parameters to have an idea about mammary health, these techniques are inadequate for determining both subclinical mastitis and milk quality (Abed et al., 2021; Tzelos et al., 2022). Moreover, it is not recommended to diagnose subclinical mastitis using a few parameters (Sumon et al., 2020). In this study, we conducted two different experiments to investigate the potential role of selected miRNAs as alternative biomarkers for subclinical mastitis and milk quality in cows.
Notable changes have been determined in electrical conductivity, SCC, and MDA levels in CMT (+) samples in Experiment I. Electrical conductivity has been suggested as a marker for mastitis detection in cattle (Cavero et al., 2006). However, in Experiment II, no differences have been found between groups, even though an increase in this parameter is associated with mastitis. On the other hand, the differences in SCC and MDA levels in the groups were similar to those observed in Experiment I, as expected (Carvalho-Sombra et al., 2021). Considering that the electrical conductivity of milk is influenced by factors such as the lactation period, this study also demonstrates that electrical conductivity is not as reliable as CMT and SCC for diagnosing subclinical mastitis. In the studies, the MDA levels in milk have been associated with mastitis, and our findings are supportive strongly of this (Carvalho-Sombra et al., 2021). Although parameters related to milk quality, such as lactose, have been reported to decrease due to infection in mammary tissue, this study has found no significant changes in compositional parameters in groups with positive CMT and microbiological analysis findings (Bobbo et al., 2017; Martins et al., 2020). It has been considered that the key factors leading to similar results in terms of milk quality parameters between the groups in both experiments may be related to the severity and stage of mastitis.
The expression levels of miR-27a-3p increased approximately 3-fold in CMT (+) samples in Experiment I, but there was no significant increase in CMT (+) and microbiological analysis results in negative samples in Experiment II. However, it was upregulated in samples that detected both Staph spp. and Strep spp. This miRNA, which relates molecular pathways such as inflammation and apoptosis (Cheng et al., 2012; Luo et al., 2020), was reported to increase mammary tissue in the presence of gram-negative bacteria in a study conducted by Wang et al. (2020).
Although miR-27a-3p showed a positive correlation with SCC and MDA, it was not directly related to milk quality parameters. However, it is considered that it can be used as a biomarker in cases of subclinical mastitis, which is pathogenic. In a study conducted in bovine mammary epithelial cells, miR-27a-3p was reported to target PPARG and could be associated with milk fat and induced by LPS, but our findings did not support this (Wang et al., 2021). It might be related to the miR-27a-3p showing cell-type-specific activity.
The significant downregulation of miR-125b in all CMT (+) groups in both experiments is consistent with similar studies in the literature (Chen et al., 2020; Luoreng et al., 2021). miR-125b, associated with natural immunity and inflammation, is reported to change levels during bacterial infection (Valmiki et al., 2020). The reduction in the response to bacterial infections is emphasized when this miRNA is silenced (Liu et al., 2020). The negative correlation between electrical conductivity and SCC in this study corroborates this information. However, since this miRNA does not have a direct relationship with milk quality parameters and is found to have lower expression levels in CMT (+) samples without bacteria, it suggests that miR-125b may not be reliable for use in subclinical mastitis detection in milk alone, as it could lead to false positive results.
The expression levels of miR-148a showed variations among groups but did not exhibit significant changes. While some studies in the literature have suggested that miR-148a could be used as a biomarker in different forms of mastitis, the results obtained from samples grouped based on both CMT and microbiological analysis in this study, indicate that miR-148a may not be a reliable biomarker for subclinical mastitis detection (Albenzio et al., 2019; Malik et al., 2018). In these relevant studies, an association between somatic cell count in milk and miR-148a is mentioned. However, the positive correlation findings between miR-148a and milk fat and MDA levels in this study make miR-148a a potential biomarker target for assessing milk quality. The positive correlation with MDA, which is an indicator of lipid peroxidation, suggests that it may affect milk quality through milk fat and fatty acid profiles.
In a study related to mastitis in cattle, similar to the findings of this study, it was reported that miR-148a did not change during mastitis but could be associated with milk quality (Sun et al., 2015). Feng et al. (2021) reported that miR-148a suppresses factors involved in adipogenesis. Based on the findings of this study and in line with the literature, miR-148a, which is stated to be involved in the regulation of more than 100 genes, is considered to be related to milk quality through milk fat. Consequently, it is believed that this miRNA might be evaluated as a biomarker in studies related to milk quality (Cai et al., 2021; Okumura et al., 2021; Quan et al., 2020).
Current research reports a limited understanding of the activities of miR-155, especially in bacterial-origin infectious diseases (Zhang et al., 2022). Studies have shown that miR-155 regulates genes and transcription factors involved in inflammation and is a major regulator of inflammation and immune response (Hu et al., 2022; Zeng et al., 2015). In bacterial infections, increased miR-155 activity in immune system cells has been reported, demonstrating activity against microbial pathogens through the IL-17/IL-23 pathway (Mirzaei et al., 2020). However, it is believed that this miRNA exhibits tissue- and agent-specific activities. In a study focused on respiratory infections caused by Strep spp., miR-155 has been indicated to play a major role in the cellular response (Verschoor et al., 2014). Another study suggested that the activity of miR-155 may decrease during the infection period, depending on the pathogen (Zhang et al., 2022). In this study, miR-155 expression levels showed a significant increase in milk infected with Strep spp., whereas there was no significant change in other groups, including the Staph spp. group compared with the control. The lack of changes in miR-155 levels in the Staph spp. group is thought to be associated with the type of tissue and the form of mastitis. In addition, as reported by Zhang et al. (2022), there is a need for a comprehensive molecular-level analysis of the effects of bacteria-specific proteins on the immune response generated through miR-155. Previous research has reported the activity of miR-155 in lymphocytes, macrophages, and mammary epithelial cells (Rodriguez et al., 2007; Wagner et al., 2013). Furthermore, researchers have identified a relationship between miR-155 and clinical mastitis (Lai et al., 2017; Srikok et al., 2020). While miR-155 alone may not be sufficient for the diagnosis of subclinical mastitis in CMT (+) samples, it might be considered a potential biomarker for detecting subclinical mastitis cases caused by Strep spp.
Although miR-155 showed a significant change only in milk samples infected with Strep spp., it was found to have a significant negative correlation with several measured quality parameters including FFDM, protein, and lactose in the study. No research investigating the relationship between miR-155 and milk quality was found in the literature. However, one study reported a relationship between ration content and miR-155 levels in milk (Abou El Qassim et al., 2022). The correlation of miR-155 with milk components such as FFDM, protein, and lactose highlights its potential as a biomarker for determining milk quality.
miR-223 was upregulated in both experiments in this study. Yet, according to the results, it is thought that the main factors for these upregulations are both Staph spp. and Strep spp. pathogens in CMT (+) milk samples. Previous research has demonstrated that miR-223 is an effective regulator in the differentiation of immune system cells and inflammation (Han et al., 2020; Jiao et al., 2022). As one of the key regulators of innate immunity, miR-223 actively participates in the induction of an inflammatory response in mammary epithelial cells in cows induced by LPS, targeting the RHOB gene (Yuan et al., 2018). It has been reported that miR-223 in cows is involved in regulating various infectious diseases such as mastitis by targeting the CXCL13 and HMGB1 genes (Fang et al., 2016; Yang et al., 2012). In this study, the increased expression of miR-223 in Staph spp. and Strep spp. sample groups is also correlated with changes in milk composition at different stages of lactation in other studies (Han et al., 2020; Wang et al., 2012). The positive correlation of miR-223 with milk fat confirms this relationship. Furthermore, the positive correlation between miR-148a, which we have associated with milk quality through milk fat and MDA, and miR-223 has enabled us to consider miR-223 as an important potential biomarker for milk quality.
As in humans, there is an ongoing search for alternative biomarkers in the monitoring, diagnosis, and treatment of diseases, as well as the assessment of productivity and quality parameters in farm animals. The investigation of miRNAs known to be active in biological fluids and highly conserved across species, isolated from milk without invasive methods, holds promise in achieving goals related to health, productivity, and quality in the livestock sector. To the best of our knowledge, there is no study that deeply explores the miRNAs in relation to pathogen-specific subclinical mastitis cases and their association with milk quality-related parameters. Although similar studies in the literature have conducted association analysis with similar sample sizes in line with our study, we believe that highly reliable findings will be obtained with new studies using a larger number of animals (Ammah et al., 2018; Tzelos., 2022).
In conclusion, we believe that this study regarding targeted miRNAs may make a significant contribution to producing data collected non-invasively on subclinical mastitis and its association with milk quality. The results of both experiments in the study particularly highlight the potential biomarker capabilities of miR-27a-3p and miR-223. These markers may be effective in diagnosing subclinical mastitis, especially those caused by prevalent Staph spp. and Strep spp., while miR-148a, miR-155, and miR-223 stand out in determining milk quality. In addition, it might be stated that miR-155 may serve as a biomarker for the diagnosis of subclinical mastitis in cattle infected with Strep spp., based on the specific increase of miR-155 in animals with subclinical mastitis infected with Strep spp. in Group IV of Experiment II. Even if significant changes were observed in the measured parameters in the milk, due to the limited size of the study sample, there is a need for further research in larger farms with a greater number of animals.
ACKNOWLEDGMENTS
This study was supported by the Hatay Mustafa Kemal University, Scientific Research Projects Unit with 22.GAP.052 Project number. In memory of our beloved friend, Asst. Prof. Dr. Erhan TEK, who contributed to the microbiological analyses in this study and whom we lost along with his dear wife in the major earthquake that occurred on February 6, 2023.
CONFLICT OF INTEREST STATEMENT
Authors declare no Conflict of Interests for this article.
ETHICAL STATEMENT
All methods and procedures strictly complied with the “Regulation on the Studying Procedures and Principles of Animal Experiments of Ethics Committees” of the Ministry of Agriculture and Forestry (2014, Republic of Turkiye) and regulations of the Animal Experiments Local Ethics Committees of Hatay Mustafa Kemal University.




