Enhancing physicochemical, rheological properties, and in vitro rumen fermentation of starch with Melastoma candidum D. Don fruit extract
Abstract
The utilization of polyphenol-modified starch in ruminants has not undergone extensive exploration. This study aimed to investigate the impact of the complex formed between starch and Melastoma candidum D. Don fruit extract on physicochemical properties, phenol release kinetics in various buffers simulating the gastrointestinal tract, methane production, and post-rumen digestibility. The interaction between starch and M. candidum D. Don fruit extract significantly (p < 0.001) increased resistant starch and particle size diameter. The maximum phenolic release from complex between starch and M. candidum D. Don fruit extract, due to gastrointestinal tract-simulated buffers, ranged from 22.96 to 34.60 mg/100 mg tannic acid equivalent. However, rumen and abomasum-simulated buffers released more phenolic content, whereas the intestine-simulated buffer showed higher antioxidant activity (ferric ion-reducing antioxidant power). Furthermore, complex between starch and M. candidum D. Don fruit extract significantly decreased dry matter rumen digestibility (p < 0.001) and maximum methane gas production (p < 0.001).
1 INTRODUCTION
Ruminants rely on starch as a crucial energy source for their metabolism. Glucose, vital for ruminants, is obtained through carbohydrate fermentation in the rumen, with starch being a major component in various feedstuffs such as grains and concentrates. While a starch-rich diet in dairy cows can enhance milk yield and composition, it has been observed to decrease methane gas production (Gómez et al., 2016). However, excessive starch intake can lead to sub-acute ruminal acidosis (Chen, Wu, et al., 2022). Therefore, optimizing starch utilization in ruminant diets is paramount for enhancing animal performance and health.
One approach to optimize starch utilization is through the incorporation of rumen undegradable starch (RUS). RUS, resistant to breakdown by rumen bacteria, passes through the rumen and is digested in the small intestine. It is a crucial component in feed as it reduces starch degradation in the rumen, thereby enhancing feed efficiency. Studies indicate that increasing the proportion of RUS in the diet can improve feed efficiency and milk production in dairy cows (Peyrat et al., 2016).
Several factors influence starch digestibility in the rumen, including the type of starch and starch modification methods. The digestibility of starch varies, with some types being more resistant to rumen fermentation than others. For instance, high-amylose starches exhibit greater resistance compared to high-amylopectin starches (Zhao et al., 2018). Modification methods, both chemical and physical, can alter starch structure to reduce digestibility.
Physical treatments like irradiation have been demonstrated to coagulate starch granules, decreasing the digestibility of rice starch (Shu et al., 2013). Autoclaving and storage at room temperature have similar effects on reducing starch digestibility (Lee et al., 2013). Chemical modification treatments, such as cross-linking, acetylation, and phosphorylation, can also decrease digestibility by altering the physicochemical properties of starch (Bensaad et al., 2022). Cross-linking achieved through intra- or intermolecular bonds at random locations in starch granules has been shown to decrease digestibility (Páramo-Calderón et al., 2016). Acetylation of retrograded potato starch has been reported to reduce digestibility to around 10% (Ziȩba et al., 2019). Another study by Niu et al. (2019) found that NaCl increased the formation of starch-lipid inclusion complexes, potentially decreasing starch digestibility. Additionally, the addition of certain compounds to starch, such as Lycium barbarum polysaccharide or phenolic compounds found in tea, has been demonstrated to decrease starch digestibility by influencing its physicochemical properties and inhibiting digestive enzymes (Gutierrez et al., 2020; Tao et al., 2023).
Limited studies have explored the utilization of modified starch as ruminant feed. According to Deckardt et al. (2013), heat–moisture treatment shows potential to increase resistant starch (RS) in feed. Additionally, parboiling treatment has been reported to enhance rice flour post-rumen digestibility by 10.43% and decrease methane production by 10.27% (Fidriyanto et al., 2021). A recent study on cassava starch revealed that heat–moisture treatment increased RS, reduced rumen digestibility, and mitigated methane production (Putra et al., 2023). A meta-analysis by Rahmadani et al. (2023) indicated that chemical modification in starch significantly decreases rumen degradable starch while increasing the slowly degradable fraction. However, there is a scarcity of literature on the use of polyphenol-modified starch in ruminants.
In this study, Melastoma candidum D. Don extract (EMCF) served as the phenolic source. M. candidum D. Don (MCF), an invasive weed commonly found in tropical and subtropical Asian countries (Ng et al., 2017; Zhang et al., 2020), was the basis for EMCF. Previous research indicated that EMCF contained 289.76 μg tannic acid equivalent (TAE)/mg total phenol and 210.07 μg quercetin equivalent (QE) /mg total flavonoid (Fidriyanto et al., 2023). In the present study, we investigated the effects of the complex between starch and EMCF on physicochemical properties, methane production, and post-rumen digestibility. Changes in starch structural properties were assessed through Fourier transform infrared (FT-IR), X-ray diffraction (XRD), and thermal gravimetric analysis (TGA). For a comprehensive understanding of the potential use of the starch-EMCF complex as ruminant feed, we explored RS, slowly digestible starch (SDS), rapidly digestible starch (RDS), starch hydrolysis kinetics, phenol release kinetics in various buffers simulating the gastrointestinal tract, methane gas production kinetics, and starch digestibility through in vitro analysis.
2 MATERIALS AND METHODS
2.1 Material
Starch was procured from Xilong Scientific Co. Ltd., China. MCF fruit was sourced from PT Perkebunan Nusantara (PTPN) VIII, Indonesia. Analytical reagent-grade chemicals were utilized in all experiments.
2.2 Preparation of M. candidum D. Don fruit extract
MCF underwent drying (oven dryer, 50°C, 48 h), grinding, and screening with a 0.5 mm screen. MCF powder was then extracted using 70% acetone in a 1:10 w/v ratio with an ultrasonicator Vibracell 75,043 (Bioblock Scientific, Illkirch, France). The ultrasonicator settings were 100% amplitude, 40 s pulse on followed by 5 s off, and a temperature of 35°C. Extraction duration was 60 min. Filtration using Whatman filter paper No. 1 (GE Healthcare UK Limited, Buckinghamshire, UK) separated the supernatant. Concentration and drying were achieved through rotary evaporators and freeze-drying, respectively. Extract powder was stored in dark bottles at −20°C.
2.3 Preparation of starch-EMCF complex
Starch-EMCF complex preparation followed the methods of Kan et al. (2022) with modifications. Twenty grams of starch were combined with EMCF levels equivalent to 0%, 5%, 10%, and 15% of the starch's dry weight. Distilled water was added in a 1:5 (w/v) ratio. Mixing occurred using a T25 digital ULTRA-TURRAX T25 (IKA, Staufen, Germany) at 5,000 rpm for 3 min, followed by a 60 min incubation in a water bath (39°C). Stirring of the mixture (5,000 rpm for 15 s) occurred every 5 min during incubation. Centrifugation (3,500×g, 10 min) separated the supernatant, which was stored for total phenolic and flavonoid analysis. The precipitate was freeze-dried, and the initial extract amounts (0%, 5%, 10%, and 15%) denoted the starch-EMCF complexes as SE-00E, SE-05E, SE-10E, and SE-15E, respectively.
2.4 Chemical analysis
The dry matter and organic matter were analyzed according to the AOAC (2016) protocols. Starch content was assessed using the Total Starch Assay Kit Megazyme (K-TSTA-100A, Neogen, US). Swelling power and solubility were analyzed according to Adebowale et al. (2002).
2.5 Bound total phenol and flavonoid
Bound total phenol and flavonoid were calculated by subtracting the free total phenol and total flavonoid in the supernatant from the initial amount in the extract. Supernatant analysis for total phenolic and flavonoid content followed the methods of Makkar (2003) and Lin and Tang (2007), respectively.
2.6 Released total phenolic
2.7 In vitro starch digestibility assay
2.8 FT-IR
Samples from each replication in the same treatment were pooled and analyzed for FT-IR. The FT-IR analysis was performed using Perkin-Elmer UATR Two (MA, USA) with wavenumbers ranging from 4,000 to 400 cm−1, resolution set at 4 cm−1, and interval data at 1 cm−1. Crystalline index and amorphous index were determined by the intensity ratio at specific wavenumbers 1,045 cm−1/1022 cm−1 (A1045/1022) and 998 cm−1/1022 cm−1 (A998/1022), respectively (Ma et al., 2021).
2.9 XRD
Where Acr is the crystalline area, and Aam is the amorphous area.
2.10 TGA
Samples from each replication in the same treatment were pooled and analyzed for TGA. TGA was conducted using Perkin-Elmer TGA 4000 instruments (MA, USA) with a temperature range of 25–500°C, an Argon flow rate of 20 ml/min, and a heat rate of 10°C/min.
2.11 Particle size analysis
Starch particle size distribution was determined following the procedure described by Pranoto et al. (2021) using a particle size analyzer Mikro–CILAS 1990 (Cilas, Centre-Val de Loire, France).
2.12 In vitro rumen fermentation
In vitro, rumen fermentation was conducted using the method developed by Theodorou et al. (1994) with modifications. The samples comprised four treatments (SE-00E, SE-05E, SE-10E, and SE-15E) with four replications. Four fistulated Ongole crossbred cattle were used as rumen fluid donors (Approved for protocol use by the ethical clearance committee BRIN No. 011/KE.02/SK/6/2022). Rumen fluid was collected before morning feeding, filtered (two-layered cheesecloth), mixed, and stored in prewarmed bottles.
Rumen and post-rumen digestibility analyses were performed by preparing two sets of samples, and both were analyzed simultaneously under the same conditions. Each sample consisted of 500 mg and 50 ml of rumen solution (rumen fluid-McDougall buffer with a ratio of 1:2[v/v]) in a 100 ml serum bottle. The bottles were flushed with CO2 (30 s), sealed, and incubated in a water bath incubator for 48 h at 39°C. Gas and methane production were monitored at specific intervals (2, 4, 6, 8, 10, 12, 24, and 48 h).
Rumen fluids were separated from the precipitate by centrifugation (6,000 rpm, 10 min, 4°C). Rumen fluids were analyzed for pH, N-NH3 (Souza et al., 2013), and volatile fatty acids (Sarwono et al., 2022). The precipitate was used to calculate rumen dry matter (R-DMD) and organic matter digestibility (R-OMD), according to Tilley and Terry (1963). For post-rumen digestibility analysis, the precipitate was mixed with 50 ml of pepsin-HCl solution and incubated at 39°C for 48 h. Total dry matter digestibility (T-DMD) and total organic matter digestibility (T-OMD) were calculated using the same methods as rumen digestibility.
2.13 Statistical analysis
The experiment was organized based on a completely randomized design. The in vitro rumen fermentation analysis was structured using a completely randomized block design. Data were subjected to one-way analysis of variance followed by Duncan's test (p < 0.05) using SPSS software, Version 23.0 (SPSS Inc., IBM, IL, USA).
3 RESULTS
3.1 Chemical properties of starch-EMCF complex
The chemical and IVSD characteristics of the starch-EMCF complex are presented in Table 1. The starch content decreased with an increase in the number of extract additions. The study revealed that the starch-EMCF complex increased swelling power (p < 0.001), with no significant difference between SE-05E, SE-10E, and SE-15E treatments. Solubility tended to increase in starch-EMCF complex treatments.
| Parameter | SE-00E | SE-05E | SE-10E | SE-15E | SEM | p-value |
|---|---|---|---|---|---|---|
| DM (%) | 95.21a | 99.67b | 99.56b | 99.63b | 0.49 | <0.001 |
| OM (%) | 95.15a | 99.62b | 99.51b | 99.59b | 0.50 | <0.001 |
| Swelling power (g/g) | 1.92 | 2.36 | 2.34 | 2.29 | 0.05 | <0.001 |
| Solubility (%) | 25.92 | 28.85 | 28.84 | 28.44 | 2.02 | 0.105 |
| Starch (g/100g DM sample) | 99.82d | 96.56c | 95.17b | 93.37a | 0.62 | <0.001 |
| Bound Total phenol (%) | 0a | 58.95b | 59.99b | 67.11c | 7.01 | <0.001 |
| Bound Total flavonoid (%) | 0a | 94.74b | 95.76c | 96.11c | 10.68 | <0.001 |
| D0 (g/100 g dry starch) | 16.95c | 13.53a | 14.24b | 14.22b | 0.35 | <0.001 |
| D∞-0 (g/100 g dry starch) | 65.31d | 55.04c | 44.11b | 34.38a | 3.01 | <0.001 |
| D0 + D∞-0 (g/100 g dry starch) | 82.25d | 68.57c | 58.35b | 48.60a | 3.24 | <0.001 |
| K x 10-3 (g min-1) | 8.23c | 7.46b | 4.26a | 4.01a | 0.49 | <0.001 |
| RDS (g/100g dry starch) | 30.04d | 22.36c | 20.57b | 18.39a | 1.13 | <0.001 |
| SDS (g/100g dry starch) | 31.87c | 33.43c | 19.16b | 13.46a | 2.22 | <0.001 |
| RS (g/100g dry starch) | 38.09a | 44.21b | 60.26c | 68.14d | 3.15 | <0.001 |
- SE-00E: Starch without MCF extract addition (control), SE-05E: Starch with 5% MCF extract addition, SE-10E: Starch with 10% MCF extract addition, SE-15E: Starch with 15% MCF extract addition, DM: Dry matter, OM: Organic Matter, D0: digested starch at t=0, D∞-0= digested starch at t= ∞ minus digested starch at t=0, D0 + D∞-0: digested starch at t= ∞, K x 10-3: starch digestion rate, RDS: Rapidly digestible Starch, SDS: Slowly digestible Starch, RS: Resistant Starch, SEM: standard error of mean, a-d Means with different superscripts within the row significantly differed (p < 0.05).
The bound total phenol value ranged from 0% to 67.11% and appeared to improve with the increased MCF extract addition. The highest bound of total phenol was observed in SE-15E treatment. The bound total flavonoid value ranged from 0% to 96.11%. There was no significant difference between bound total flavonoids on SE-10E (95.76%) and SE-15E (96.11%). No bound total phenol and flavonoid were found in SE-00E treatments since no extract was added.
3.2 IVSD of starch-EMCF complex
The changes in RDS, SDS, and RS followed the decrease in starch digestibility due to MCF extract addition. RDS and SDS significantly (p < 0.001) decreased with the increase of MCF extract. The addition of 5%, 10%, and 15% MCF extract decreased RDS by 25.57%, 31.52%, and 38.77%, respectively. The addition of 5% MCF extract did not affect SDS but significantly (p < 0.001) increased RS content by 6.12%. Furthermore, the highest RS was observed with the addition of 15% MCF extract with 68.14 g/100 g dry starch.
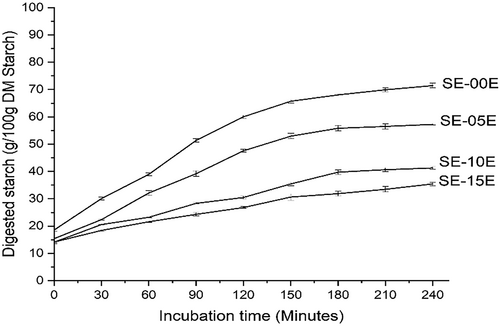
The effect of MCF extract on starch digestibility was investigated (Figure 1). The starch-EMCF complex exhibited slow digestion from the beginning of incubation. After 240 min, the SE-15E treatment decreased DS by 50.40% compared to the control. The non-linear model was fitted to the data, and the estimated DS at t = ∞ and starch digestion rate (K) are shown in Table 1. Concerning the kinetics of starch digestibility, this study showed that DS at t = 0 (D0) decreased in the MCF extract treatment compared to the control. The lowest D0 was observed in SE-05E (13.53 g/100 g dry starch) and increased with the extract addition. Moreover, there was no significant difference between D0 in SE-10E and SE-15E treatments. D∞-0 significantly decreased with the increase of the extract addition. The same trend was observed in D0 + D∞-0 parameter. Compared to the control, SE-15E decreased D∞-0 and D0 + D∞-0 by 47.36% and 40.91%, respectively. The addition of 10% and 15% of MCF extract showed the lowest starch hydrolysis rate with 4.26 × 10−3 g min−1 and 4.01 × 10−3 g min−1, respectively.
3.3 The structure of starch-EMCF complex
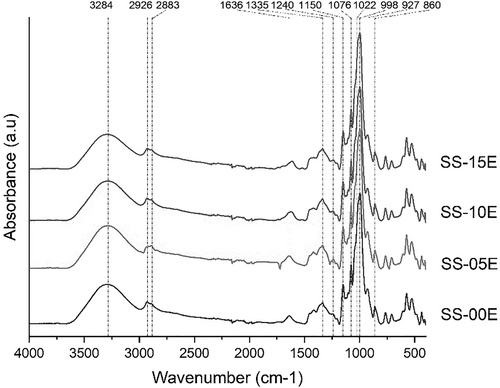
FT-IR, a widely used analytical technique for studying starch structure, revealed that the complex starch-MCF extract exhibited similar peaks with minor intensity variations compared to the control (Figure 2). The peak around 3,290 cm−1 in the control shifted to a lower wavenumber in the complex, indicating a more potent hydrogen bonding interaction between starch and phenolic (Liu et al., 2017). The peak around 2,930 cm−1 was related to -CH2 stretching vibration on starch (Pang et al., 2011), and the peak around 1,630 cm−1 indicated the stretching vibration of -OH groups, signifying the amorphous region of starch (Wang et al., 2015). The region between 1,500 and 1,200 cm−1 featured deformational vibrations of local symmetry groups like CH2 and various C-OH deformations observed in carbohydrates. The fingerprint area of starch, between 1,200 and 800 cm−1, displayed peaks at 1335, 1240, and 1,150 cm−1, signifying CH2, CH2OH (side chain), and C-O or C-C stretching vibrations, respectively (Shivaraju et al., 2019). The peak at 1076 cm−1 indicated O-H bond stretching of C-O-C glycosidic linkages (Liu et al., 2002). Additionally, peaks around 1,022 cm−1 (C–O–H deformation) and 998 cm−1 (C-O of C-O-C) were related to the characteristic amorphous region in starch (Shivaraju et al., 2019; Van Soest et al., 1995). Peaks around 926 and 861 cm−1 were related to the skeletal mode vibration of α-1,4 glycosidic linkage (C-O-C) and CH2 deformation, respectively (Yao et al., 2002).
The starch crystallinity, as shown in Table 2 and X-ray diffractograms (Figure S1), exhibited an A-type diffraction pattern (Figure S1) with strong peaks at 2θ, close to 15°, 17°, and 23°. Starch crystallinity varied from 9.08% to 30.04%, with a trend toward increasing crystallinity with higher extract additions.
| XRD | FTIR | |||
|---|---|---|---|---|
| Treatments | Crystallinity (%) | Amorphous (%) | Crystalline index (A1045/A1022) | Amorphous index (A998/A1022) |
| SE-00E | 9.08 | 90.92 | 0.72 | 1.20 |
| SE-05E | 10.27 | 89.73 | 0.73 | 1.21 |
| SE-10E | 27.98 | 72.02 | 0.73 | 1.19 |
| SE-15E | 30.04 | 69.96 | 0.74 | 1.15 |
- SE-00E: Starch without MCF extract addition (control), SE-05E: Starch with 5% MCF extract addition, SE-10E: Starch with 10% MCF extract addition, SE-15E: Starch with 15% MCF extract addition.
Particle diameter (D10, D50, and D90) and the span factor (Table 3) showed that the average particle size was 14.40–17.26 μm. The starch-MCF extract complex increased the average particle size diameter and particle size dispersion (span). The largest average particle size and span were observed in SE-15E with 17.26 and 1.61 μm, respectively. The increase in the span was due to changes in the particle diameter in D10, D50, and D90. The particle diameter at 10% cumulative distribution (D10) significantly decreased (p < 0.001) in starch treated with MCF extract. The particle size diameters D50 and D90 showed the opposite trend with D10, increasing with the addition of MCF extract. The highest (p < 0.001) particle size increases at D50 and D90 were observed in SE-15E with 16.48 and 30.21 μm, respectively.
| Treatments | D10 (μm) | D50 (μm) | D90 (μm) | Average diameter (μm) | Span |
|---|---|---|---|---|---|
| SE-00E | 4.06c | 14.59a | 22.16a | 14.40a | 1.24a |
| SE-05E | 3.55a | 16.03b | 27.08b | 16.18b | 1.47b |
| SE-10E | 3.63ab | 16.19b | 28.69c | 16.69c | 1.54c |
| SE-15E | 3.67b | 16.48c | 30.21d | 17.26d | 1.61d |
| SEM | 0.05 | 0.19 | 0.79 | 0.28 | 0.04 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
- SE-00E: Starch without MCF extract addition (control), SE-05E: Starch with 5% MCF extract addition, SE-10E: Starch with 10% MCF extract addition, SE-15E: Starch with 15% MCF extract addition, D10, D50, and D90 are the particle diameters corresponding to 10%, 50%, and 90% of the cumulative distribution, respectively. Span is indicative of the particle size dispersion. SEM: standard error of mean, a-d Means with different superscripts within the column significantly differed (p < 0.05).
3.4 Thermogravimetric analysis of starch-EMCF complex
During the thermal degradation of starch, dehydration, and decomposition are typically observed in two phases. All treatments exhibited two stages of weight loss, as shown in Figure S2. The initial stage of weight loss, between 35°C and 120°C, is attributed to the evaporation of moisture content and volatile compounds. The second phase of weight loss occurred between 280°C and 340°C, with a significant mass decrease. Starch without MCF extract addition had a higher weight loss of 70.48%. SE-00E had the highest maximum temperature transition (316.33°C) and final temperature (339.86°C). Increasing MCF extract addition showed a decreasing trend of initial, maximum, and final transition temperatures. Moreover, increasing MCF extract addition to starch decreased weight loss during the second degradation phase. The highest weight loss in SE-15E decreased by 15.37% compared with SE-00E, followed by SE-10E (14.10%) and SE-05E (8.54%), respectively. There is an increasing trend of residual weight at 500°C with the increase in the addition of extract (Table 4). Starch with 15% MCF extract addition (SE-15E) increased the residual weight by 52.48% compared with SE-00E, followed by SE-10E (39.12%) and SE-05E (14.42%).
| Treatments | Transition temperatures | Weight lost at corresponding transition (%) | Residual weight (%) at 500°C | ||
|---|---|---|---|---|---|
| Ti (°C) | Tm (°C) | Tf (°C) | |||
| SE-00E | 290.19 | 316.33 | 339.86 | 70.48 | 15.95 |
| SE-05E | 290.40 | 303.45 | 335.96 | 64.46 | 18.25 |
| SE-10E | 283.91 | 310.11 | 333.15 | 60.54 | 22.19 |
| SE-15E | 280.11 | 305.54 | 330.41 | 59.65 | 24.32 |
- SE-00E: Starch without MCF extract addition (control), SE-05E: Starch with 5% MCF extract addition, SE-10E: Starch with 10% MCF extract addition, SE-15E: Starch with 15% MCF extract addition, Ti: Initial temperature for transition, Tm: Maximum temperature for transition, Tf: Final temperature for transition
3.5 Released phenolic from starch-EMCF complex
The total phenol release kinetics of the starch-EMCF complex were analyzed using the Gompertz non-linear model (Table 5). The results indicated a positive lag time value for all samples in all buffers, suggesting a delayed release of phenol from starch. The lag time showed a tendency to decrease with an increase in the extract added. The longest lag time occurred in the SE-05E treatment at 4.29 h, and this value increased as the buffer's pH decreased. There was a significant decrease in the amount of phenol released (p < 0.001) with an increase in extract concentration in all buffers. In the phosphate buffer (pH 7.4), the SE-05E treatment released 34.60 mg/100 mg TAE, significantly decreasing (p = 0.006) in SE-10E (23.08 mg/100 mg TAE) and SE-15E (22.96 mg/100 mg TAE). The lowest phenol release occurred at pH 7.4 in the SE-10E and SE-15E treatments. Rumen-simulated and abomasum-simulated buffers released more phenol compared to the small intestine-simulated buffer.
| Parameter | Free phenolic | SEM | p-value | ||
|---|---|---|---|---|---|
| SE-05E | SE-10E | SE-15E | |||
| Acetate buffer pH 5.6 | |||||
| L (hour) | 3.19b | 1.89a | 1.44a | 0.24 | <0.001 |
| B (mg/100mg TAE) | 32.74b | 31.08b | 25.28a | 1.06 | <0.001 |
| c (mg TAE/ hour) | 0.41 | 0.31 | 0.27 | 0.03 | 0.142 |
| Citrate buffer pH 2.9 | |||||
| L (hour) | 2.12b | 1.49a | 1.40a | 0.11 | 0.003 |
| B (mg/100mg TAE) | 32.78b | 34.42b | 25.90a | 1.16 | <0.001 |
| c (mg TAE/hour) | 0.59 | 0.60 | 0.63 | 0.02 | 0.638 |
| Phosphate buffer pH 7.4 | |||||
| L (hour) | 4.29c | 1.32a | 2.57b | 0.38 | <0.001 |
| B (mg/100mg TAE) | 34.60b | 23.08a | 22.96a | 2.00 | 0.006 |
| c (mg TAE/ hour) | 0.35b | 0.20a | 0.34b | 0.02 | <0.001 |
- SE-00E: Starch without MCF extract addition (control), SE-05E: Starch with 5% MCF extract addition, SE-10E: Starch with 10% MCF extract addition, SE-15E: Starch with 15% MCF extract addition, L: lag time, B: Maximum total phenolic released, c: rate of total phenolic released, TAE: Tannic acid equivalent, SEM: standard error of mean, a-d Means with different superscripts within the row significantly differed (p < 0.05).
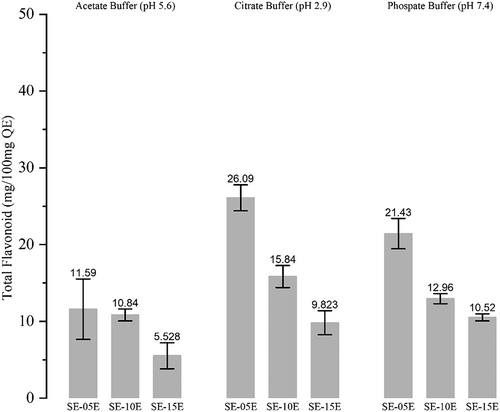
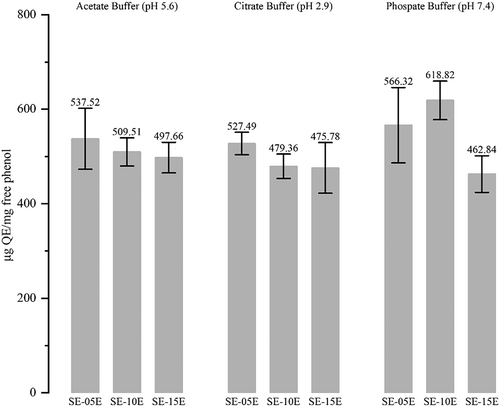
The antioxidant activity of released total phenol was analyzed with FRAP analysis, showing that phenolic compounds released in all buffers exhibited antioxidant activity (Figure 4). The highest FRAP value was observed in the small-intestine-simulated buffer (pH 7.4). Although the total phenol released in phosphate buffers was low, the antioxidant activity was higher than in other buffers. The antioxidant activity of phenolic compounds released in rumen-simulated and abomasum-simulated buffers was almost similar. However, the amount of total phenol and flavonoid released was higher in the citrate buffer (Figure 3).
3.6 In vitro rumen fermentation profiles of starch-EMCF complex
The in vitro rumen fermentation profile of the starch-EMCF complex is presented in Table 6. The results showed that the formation of a complex between starch and MCF extract significantly (p < 0.001) inhibited gas production at all sampling points during rumen incubation in vitro. The greater the MCF concentration in a starch-EMCF complex, the greater the suppression of gas production. The gas inhibition effect decreased along with the longer incubation time, with inhibition levels ranging from 20.59–35.04% at 24 h and 6.29–15.62% at 48 h compared to the control. Gas kinetic analysis revealed that the starch-EMCF complex increased the maximum total gas production potential by 33.72–80.13% more than the control, and the gas production rate was significantly lower in the starch-EMCF complex than in the control.
| Parameter | SE-00E | SE-05E | SE-10E | SE-15E | SEM | p-value |
|---|---|---|---|---|---|---|
| Gas production (ml) | ||||||
| 2 h | 15.63c | 7.75b | 6.63ab | 5.75a | 1.08 | <0.001 |
| 4 h | 32.31c | 15.25b | 13.44ab | 11.88a | 2.18 | <0.001 |
| 6 h | 50.49d | 23.50c | 21.00b | 18.63a | 3.39 | <0.001 |
| 8 h | 67.74d | 34.25c | 30.25b | 26.63a | 4.32 | <0.001 |
| 10 h | 81.86d | 46.44c | 39.69b | 34.56a | 4.89 | <0.001 |
| 12 h | 93.80d | 57.13c | 49.25b | 43.13a | 5.23 | <0.001 |
| 24 h | 145.30d | 115.38c | 103.19b | 94.38a | 5.13 | <0.001 |
| 48 h | 189.61d | 177.69c | 166.00b | 160.00a | 3.09 | <0.001 |
| a+b (ml) | 206.33a | 275.90b | 300.24b | 371.66c | 17.97 | 0.002 |
| c (ml/h) | 0.053c | 0.023b | 0.018b | 0.013a | 0.004 | <0.001 |
| Methane gas production (mg/g DM) | ||||||
| 2 h | 10.65c | 3.24b | 2.56a | 2.18a | 0.91 | <0.001 |
| 4 h | 27.16c | 7.45b | 6.05ab | 4.72a | 2.39 | <0.001 |
| 6 h | 39.62c | 11.66b | 9.50ab | 7.60a | 3.40 | <0.001 |
| 8 h | 48.41c | 15.97b | 12.99ab | 10.49a | 4.01 | <0.001 |
| 10 h | 54.05c | 18.72b | 15.43ab | 12.29a | 4.39 | <0.001 |
| 12 h | 60.09c | 22.48b | 18.19a | 14.66a | 4.75 | <0.001 |
| 24 h | 76.33c | 32.38b | 26.53a | 22.86a | 5.61 | <0.001 |
| 48 h | 89.24c | 39.67b | 32.56a | 27.72a | 6.40 | <0.001 |
| a+b (mg) | 87.61c | 41.31b | 33.95a | 29.58a | 6.04 | <0.001 |
| c (mg/h) | 0.103b | 0.068a | 0.067a | 0.062a | 0.004 | <0.001 |
| In vitro rumen digestibility | ||||||
| R-DMD (%) | 93.56d | 85.43b | 81.20ab | 77.95a | 1.67 | <0.001 |
| T-DMD (%) | 94.68d | 85.43c | 81.51b | 78.34a | 1.62 | <0.001 |
| R-OMD (%) | 96.21c | 85.16b | 79.79ab | 74.55a | 2.26 | <0.001 |
| T-OMD (%) | 97.50c | 87.06b | 81.63a | 78.52a | 1.95 | <0.001 |
| pH | 6.14a | 6.20ab | 6.24c | 6.24c | 0.02 | 0.083 |
| NH3-N (mM) | 2.96b | 2.09a | 1.95a | 2.28a | 0.16 | 0.017 |
- SE-00E: Starch without MCF extract addition (control), SE-05E: Starch with 5% MCF extract addition, SE-10E: Starch with 10% MCF extract addition, SE-15E: Starch with 15% MCF extract addition, SEM: standard error of mean, a+b: potential gas production, c: rate of gas production, R-DMD: Rumen in vitro dry matter degradability, R-OMD: Rumen in vitro organic matter degradability, T-DMD: Total in vitro dry matter digestibility (rumen + post-rumen digestibility), T-OMD: Total in vitro organic matter digestibility (rumen + post-rumen digestibility).
- SEM: standard error of mean, a-d Means with different superscripts within the row significantly differed (p < 0.05)
The starch-EMCF complex significantly reduced methane production in in vitro rumen fermentation (p < 0.001). The effect of the starch-EMCF complex was dose-dependent, with higher MCF extract doses leading to lower methane production. Methane production was inhibited by 55.55–68.94% compared to the control after 48 h of incubation. The starch-EMCF complex also lowered total methane production potential and decreased the rate of methane gas production.
The complex starch-MCF extract significantly decreased (p < 0.001) both R-DMD and R-OMD, with the highest decrease observed in SE-15E by 16.68% and 22.51%, respectively. Furthermore, the SE-15E treatment showed the highest decrease in total dry matter and organic matter digestibility by 17.26% and 19.47%, respectively.
The starch-EMCF complex demonstrated the ability to alter rumen fermentation profiles, including lowering pH and NH3-N of the rumen fluids. VFA data indicated an increase in the concentration of C2 and C3 but a decrease in C4 (p < 0.001). The change in VFA production pattern was evident in the lowered C2/C3 ratio. All treatments lowered the C2/C3 ratio, and total VFA production was significantly reduced in the starch-MCF extract by 17.86–29.96% compared with the control (Table 7).
| Parameter | SE-00E | SE-05E | SE-10E | SE-15E | SEM | p-value |
|---|---|---|---|---|---|---|
| Volatile fatty acid (%) | ||||||
| C2 | 64.20a | 69.61ab | 67.82b | 68.53b | 0.73 | 0.067 |
| C3 | 21.70a | 25.88b | 28.73bc | 29.19c | 0.88 | <0.001 |
| i-C4 | 0.83bc | 0.99c | 0.75b | 0.47a | 0.05 | <0.001 |
| C4 | 12.53e | 2.72b | 1.90ab | 1.29a | 1.20 | <0.001 |
| i-C5 | 0.63b | 0.63b | 0.55b | 0.34a | 0.04 | 0.002 |
| C5 | 0.11a | 0.17a | 0.25b | 0.17a | 0.02 | 0.011 |
| TVFA (mM) | 120.58b | 99.04a | 84.46a | 86.48a | 4.54 | 0.004 |
| C2/C3 | 2.96d | 2.69cd | 2.40bc | 2.35b | 0.08 | <0.001 |
- SE-00E: Starch without MCF extract addition (control), SE-05E: Starch with 5% MCF extract addition, SE-10E: Starch with 10% MCF extract addition, SE-15E: Starch with 15% MCF extract addition, SEM: standard error of mean, C2: acetate, C3: propionate, C4: butyrate, C5: valerate, i-C4:iso-butyrate, i-C5: iso-valerate, TVFA: total volatile fatty acids, and C2/C3: ratio of acetate to propionate, SEM: standard error of mean, a-d Means with different superscripts within the row significantly differed (p < 0.05)
4 DISCUSSION
4.1 Physicochemical changes in starch-EMCF complex
Phenolic compounds have a known capacity to interact with starch, altering its physicochemical properties. The formation of a complex between starch and polyphenols is influenced by various factors, including starch characteristics and the type of polyphenol. Starch characteristics, encompassing morphology, chemical composition, and molecular architecture, play a crucial role in this interaction (Liu, Le Bourvellec, et al., 2020). Our study demonstrated an increased solubility in starch-EMCF complexes. This aligns with findings in other complex starch-phenolic systems, such as wheat starch-tannic acid complexes (Kan et al., 2022), rice starch with various phenolic acids, and maize amylopectin with phenolic acids (Li et al., 2018), indicating enhanced swelling power and solubility compared to the control. The heightened solubility and swelling power may be attributed to the penetration of phenolic components into starch granules, interacting with amorphous areas of amylose and non-ordered amylopectin branches, consequently augmenting swelling power and amylose leaching (Kan et al., 2022).
The structure of starch is a crucial factor affecting its digestibility. Our results revealed that the complex formation between starch and EMF decreased the maximum hydrolyzed starch by enzymes compared to the control (Table 1). Similar effects were observed by Tangkhawanit and Siriamornpun (2022) in dried soy residues treated with phenolic and flavonoid compounds, resulting in reduced starch digestion from 6.6% to 2.7%. Additionally, the starch-EMCF complex significantly decreased the starch hydrolysis rate. Phenolic compounds in tea have also been reported to inhibit digestive enzymes for starch, impacting the rate of starch hydrolysis (Gutierrez et al., 2020).
Phenolic compounds exerted an influence on starch digestibility, particularly in terms of RDS, SDS, and RS (Table 1). The complex formed between polyphenols and starch granules may partially inhibit the accessibility of hydrolyzing enzymes to starch chains, thereby reducing starch digestibility and digestion rate (Kan et al., 2022). Similar effects were observed by Lemlioglu-Austin et al. (2012), who found that sorghum bran extracts slowed starch digestion and increased RS content. Moreover, a review by Van Ngo et al. (2022) highlighted the potential applications of polyphenol-modified starches in the industry due to their ability to increase levels of SDS and RS.
In starch, FT-IR absorption peaks near 1,045, 1,020, and 995 cm−1 are associated with the ordered structure of crystal regions, amorphous regions, and intramolecular hydrogen bonding of the hydroxyl group at C-6, respectively (Shigel, 2002; Van Soest et al., 1995). In our study, the value of A1045/1022 slightly increased and A998/1022 decreased with increasing MCF extract (Table 2). These results suggest an increase in the crystal phase structure of starch, a decrease in the distance between starch molecules, and a reduction in the formation of hydrogen bonds between starch molecules. Consistent with Lu et al. (2018), the relative crystallinity from XRD and the crystallinity index from FT-IR (A995/A1022 and A1045/A1022) were positively correlated with RS of raw or cooked flour samples and negatively correlated with RDS.
The XRD results (Figure S1) revealed no changes in the type of starch structure following the complexation with EMCF. Phenolic compounds, such as those found in EMCF, are known to modify starch by forming inclusion complexes with amylose molecules and binding to the side chains of amylopectin and the amorphous region of starch granules (Kandil et al., 2012). The interaction between starch and phenolic compounds can result in two types of complexes: V-type and non-V-type (Zhu, 2015). A previous study showed that the complex between starch and phenolic compounds can change the structure from A- to V-type, depending on the type of phenolic compounds, while in some cases, the structure remains in the A-type form (Zheng et al., 2020). Recent research on anthocyanins with rice starch and tartary buckwheat starch with flavonoids, under high hydrostatic pressure treatment, did not affect the starch structure and preserved its A-type structure (Miao et al., 2021; Zhou et al., 2022). In contrast, the complexation of starch with gallic acid, tannin, and proanthocyanidins changed the starch structure from A- to V-type (Amoako & Awika, 2016; Liu et al., 2019). These structural changes are driven by non-covalent interactions, significantly impacting the physicochemical properties of starch, including thermal stability, complex structure, and digestibility.
The starch crystallinity in our study varied from 9.08% to 30.04%, with a trend toward increasing crystallinity with higher EMCF extract additions. Similarly, a study by Tarazi Riess et al. (2022) on the complex between high-amylose corn starch and curcuminoid and capsaicinoids also increased starch crystallinity by 49.69% and 50.10%, respectively.
The interaction between EMCF and starch in our study decreased the degradation temperature, decreased weight loss, and increased residual weight (Table 4). This is consistent with findings by Liu, Zhong, et al. (2020) in a study on the complex between maize starch, quercetin, and epigallocatechin gallate, where the maximum temperature decreased. The reduction in degradation temperature may result from the inhibition of intramolecular and intermolecular hydrogen bonds between starches due to the large molecules of phenolic compounds, leading to decreased bond strength and decreased thermal stability. The decrease in degradation temperature was associated with the decrease in RDS and SDS, followed by an increase in RS content. This increase in RS content may contribute to decreased weight loss and increased residual weight after 500°C.
All samples exhibited bimodal particle size distribution (Figure S3), consistent with a previous study showing that native starch has a bimodal particle size distribution (Ma et al., 2022). The binding between phenolic compounds and potato and cassava starch has been found to increase starch particle size (Alves et al., 2022). Cross-linking between amyloses may contribute to increased particle size and span due to the presence of phenol. Polyphenols can form numerous connections between starch molecules, causing particle size to increase (Chai et al., 2013). The mechanisms of starch-phenolic interaction may involve covalent or non-covalent interactions. Phenolic substances, with one or more hydroxyl groups, can interact with starch to form complexes, modifying the starch's properties and affecting its structure and digestibility. Non-covalent interactions, including hydrogen bonds, van der Waals forces, and electrostatic interactions, have been identified between starch and phenolics, making starch more resistant to digestion (Liu et al., 2022). Phenolic compounds with more hydroxyl groups and higher molecular weight exhibit more potent binding effects on macromolecules, such as polysaccharides and proteins, than simple phenols (Sun et al., 2019). By forming hydrogen bonds, phenols with more hydroxyl groups and higher molecular weight can bridge or aggregate starch molecules, surrounding them with phenol as the core (Chen, Feng, et al., 2022).
4.2 Phenolic release kinetics from starch-EMCF complex
The release rate of phenols demonstrated a consistent trend with no significant variation with an increase in the amount of EMCF extract (Table 5). Phenolic compounds with more hydroxyl groups and higher molecular weight exhibited stronger binding affinity, making their release more challenging (Chen, Feng, et al., 2022). High-molecular-weight phenolic compounds, such as tannins, are known for their robust binding forces to macromolecules like proteins, structural carbohydrates, and starch, leading to decreased ruminal degradation (Besharati et al., 2022). Studies have indicated that flavonoids like rutin and quercetin may have weaker binding forces on starch than in the iodine-starch complex, although they can still alter the physicochemical properties of starch (He et al., 2018). Additionally, flavonoids conjugated with sugars and acetylated with hydroxycinnamic acids showed lower binding forces than aglycones (Quirós-Sauceda et al., 2014).
Comparing our findings to previous reports is challenging due to the limited literature on sequential phenol release from complex forms with starch in different ruminant gastrointestinal tract simulated buffers. Previous studies have focused on the ability of phenolic compounds to interact with other macromolecules, such as lipids and polysaccharides, for encapsulating phenolic compounds to enhance their release in the post-rumen and intestine. For instance, Adejoro et al. (2019) observed a burst release pattern of acacia tannin extract loaded with starch within the first 4 h, with a slower release after 8 h across three different pH buffers. Tolve et al. (2021) microencapsulated Quebracho extract with maltodextrin-gum Arabic and noted a substantial reduction in phenolic release under simulated rumen (pH 5.6) and abomasum (pH 2.2) conditions.
The released phenolic compounds from the starch-EMCF complex exhibited antioxidant activity. The antioxidant activity varied among the three different buffers, confirming the influence of environmental pH on antioxidant activity. The effect of pH on antioxidant activity depends on the specific phenolic compounds, with some showing increased activity at neutral pH and others at alkaline pH. For example, Reddy Palvai et al. (2014) found that the antioxidant activity of Canthium parviflorum and Abrus precatorius leaves extract was stable at pH 7 and decreased at pH 4.5 and 9. De La Coba et al. (2009) observed increased antioxidant activity of mycosporine-like amino acids at pH 6–8.5. Amorati et al. (2006) reported higher antioxidant activity of certain phenolic compounds at alkaline conditions (pH 8) than at acidic conditions (pH 4), and Yu et al. (2022) found that phenolic extracts from navel orange peel showed the strongest 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity at pH 7. The pH-induced alterations in antioxidant activity are attributed to changes in pKa values, corresponding to variations in the ionization of hydroxyl groups or other functional groups in phenolic compounds (Amorati et al., 2006).
4.3 In vitro rumen fermentation profiles of starch-EMCF complex
We observed a reduction in gas production during incubation with complex starch-phenolic treatments compared to the control. However, after fitting the data to a non-linear regression model by Orskov and McDonald (1979), an increased maximum gas production (a + b) was noted. Gas production profiles in vitro rumen fermentation analysis typically exhibit three stages: the lag stage (slow gas production rate), the exponential stage (fast gas production rate), and the stationary stage (diminishing gas production rate) (Dhanoa et al., 2021). The increased maximum gas production in the complex starch and MCF extract treatment may be attributed to the gas production being in the exponential stage at the end of incubation (48 h), while the control was in the stationary stage, resulting in higher maximum gas production as fitted to the non-linear regression model.
The starch-EMCF complex demonstrated the ability to inhibit methane production, potentially linked to reduced digestibility. Although the methanogen population was not measured, the study indicated that the starch-EMCF complex indirectly suppressed methane production through different mechanisms. Methane production was inhibited by lowering carbohydrate rumen digestibility, leading to a reduced availability of CO2 and H2 in the rumen for CH4 production. This aligns with a previous study by Sarwono et al. (2019), suggesting that the addition of phenolic compounds decreases CH4 by inhibiting digestibility. CH4 production in the rumen is closely tied to dietary properties, such as the amount of H2 generated during digestion (Janssen, 2010; Sarwono et al., 2018). Starch digestion by amylolytic bacteria, resulting in higher propionate production, tends to create less methane in feeds with high-amylose content. Propionate production in the rumen consumes H2, thereby reducing the supply of H2 for CH4 production.
The complex starch-MCF extract suppressed gas production by reducing digestibility, as indicated by the decreased R-DMD and T-DMD. Phenolic compounds from MCF extract were utilized in this study to protect starch from rumen degradation, a known factor that decreases starch digestibility in the rumen (Fitri et al., 2022). Martínez et al. (2005) reported that tannic acid could decrease barley rumen digestibility while improving starch utilization in ruminants. However, Mahmoudzadeh et al. (1989) found that ferulic and p-coumaric acids did not significantly affect post-ruminal digestion of starch in lambs. The reduced digestibility observed with the starch-EMCF complex may be related to changes in starch digestibility characteristics, including a decrease in RDS and SDS content and an increase in RS. RS, known to inhibit rumen digestibility, tends to protect starch from rumen degradation, enhancing the benefits of antioxidants and improving their availability and utilization when released in the post-rumen or small intestine (Fidriyanto et al., 2021; Putra et al., 2023). Starch can be categorized into three classes based on enzymatic digestibility: RDS, SDS, and RS (Zhang & Hamaker, 2009). In ruminant nutrition, RS resists ruminal degradation mechanisms, such as amylolytic bacteria and protozoa, and is primarily converted to glucose and absorbed in the small intestine (Deckardt et al., 2013; Iqbal et al., 2009). As the digestibility of starch-polyphenol complexes decreases, polyphenols are released in the post-rumen or small intestine, enhancing their bioaccessibility and health benefits. Increased post-rumen and small intestine digestibility not only protects starch from rumen degradation but also enhances the benefits of antioxidants, improving their overall availability and utilization.
The starch-EMCF complex demonstrated an increase in the production of acetic acid (C2) and propionic acid (C3), leading to a lowered C2/C3 ratio. This contradicts previous studies suggesting that the addition of starch increased propionate and decreased acetate (Gómez et al., 2016). The elevated acetate production may be attributed to the higher RS content in the starch-MCF extract complex, as demonstrated by Putra et al. (2023), who showed that RS might increase acetate production in the rumen. Additionally, the starch-EMCF complex lowered ammonia (NH3-N) concentration in the rumen in vitro, and this reduction was dose-dependent, with higher levels of phenolic compounds leading to lower NH3-N concentration. Phenolic compounds are known to form fiber-tannin and protein-tannin complex bonds with feed materials (Woodward et al., 2002), resisting fermentation by proteolytic bacteria and resulting in lower NH3-N concentration (Yanza et al., 2021).
The complex starch-EMCF was found to increase RS and decrease starch rumen digestibility. RUS has demonstrated advantages in ruminant nutrition by manipulating the digestion site of starch in the rumen. This manipulation provides health and metabolic benefits, including a reduced risk of acidosis in the rumen, increased energy utilization efficiency through enhanced net hepatic glucose supply, and inhibition of rumen methanogenesis (Deckardt et al., 2013; Putra et al., 2023). However, it should be noted that an excessive use of RUS may reduce energy availability for rumen microbial synthesis, leading to hindgut acidosis if not carefully managed. Therefore, a balanced ration incorporating other feed sources is crucial. This study is significant as it successfully elucidated the release kinetics of the starch-MCF extract complex and its effects on rumen fermentation, providing valuable information for application in the ruminant livestock industry.
In summary, the interaction between starch and MCF extract decreased rumen digestibility and methane gas production while increasing crystallinity, RS, and post-rumen digestibility in vitro analyses. The released phenolic compounds from the starch-EMCF complex, due to simulated gastrointestinal tract buffers, exhibited antioxidant activity (FRAP). Interestingly, rumen- and abomasum-simulated buffers released more phenols from the starch-EMCF complex than the small intestine-simulated buffer.
ACKNOWLEDGMENTS
This work was funded by the grants “DIPA 2023 ORHL” National Research and Innovation Agency (BRIN) No. 39/III.5/HK/2022 and RIIM BRIN-LPDP No. B-803/7.5/FR/6/2022 and B-1373/III.5/PR.03.08/6/2022. The authors acknowledge the facilities, scientific, and technical support from BRIN through E-Layanan Sains Ilab & Genomic Cibinong, Badan Riset dan Inovasi Nasional.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.




