Vitrification of immature oocytes in pigs
Abstract
Cryopreservation of oocytes is an important technology for the in vitro gene banking of female germplasm. Although slow freezing is not feasible, porcine oocytes survive vitrification at high rates. Cryopreservation at the germinal vesicle stage appears to be more advantageous than that at the metaphase-II stage. Several factors are considered to affect the success of vitrification and subsequent utilization of immature porcine oocytes such as the device, the protocols for cryoprotectant application, warming, and the post-warming culture. Although live piglets could be obtained from vitrified immature oocytes, their competence to develop to the blastocyst stage is still reduced compared to their non-vitrified counterparts, indicating that there is room for further improvement. Vitrified oocytes suffer various types of damage and alteration which may reduce their developmental ability. Some of these can recover to some extent during subsequent culture, such as the damage of the cytoskeleton and mitochondria. Others such as premature nuclear progression, DNA damage and epigenetic alterations will require further research to be clarified and addressed. To date, the practical application of oocyte vitrification in pigs has been confined to the gene banking of a few native breeds.
1 WHY PRESERVE PORCINE OOCYTES?
Cryopreservation of oocytes is an important strategy for in vitro gene banking of female germplasm safe from epidemic threats. In humans, matured oocytes are routinely cryopreserved for fertility preservation at clinics either by slow freezing or vitrification with high success rates (Rienzi et al., 2017; Sciorio et al., 2023). In farm animals, oocyte cryopreservation can be advantageous via two different ways (Figure 1). First, it can serve the purpose of gene banking for endangered breeds/strains with high economic and/or scientific value. Pigs used for human medical research are typically such animals. Many of the indigenous breeds used as model animals have limited distribution area (Chang et al., 2009), which can even be accompanied by limited reproductive potential (Grupen et al., 2019). Genetically modified (GM) pigs are also produced for human biomedical research (Hryhorowicz et al., 2020). Furthermore, certain local breeds with limited distribution area are used for premium quality meat products thus represent high economic value such as the Japanese Agu (Touma & Oyadomari, 2020), the Hungarian Mangalica (Egerszegi et al., 2003; Ratky et al., 2007) and the Spanish Iberico (Daza et al., 2008). The recent outbreaks of the African swine fever (ASF) threaten the genetic diversity of pigs worldwide and the very existence of some local breeds (Ba et al., 2020; Jiang et al., 2022). For this reason, in vitro preservation of porcine genetic resources is a currently important task.
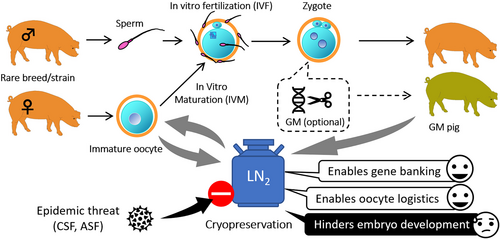
The other theoretical advantage of oocyte cryopreservation in farm animals is the extended possibilities for logistics. Cryopreserved oocytes can be stored, moved, and utilized at any time and in any place. Therefore, for technologies which require oocytes as basic materials such as the production of GM animals, it offers the possibility to be conducted irrespective of the time and place of the acquisition of the oocytes or even to temporary suspend running projects instead of cancelation (which usually means a waste of money) in case of unexpected trouble (vis major). Furthermore, cryopreserved oocytes can be stored to accumulate their numbers to levels which allow the conduction of high scale experiments. Thus, oocyte cryopreservation could facilitate a flexible planning of experiments in time and space and improve cost-efficacy.
Since embryo transfer and in vitro embryo production (IVEP) technologies have not been widely applied in pork industry, the potentials of oocyte cryopreservation in pigs have not gained as much interest as in cattle. Furthermore, porcine oocytes are more sensitive to cryopreservation procedures than oocytes of other farm animals (Mullen & Fahy, 2012). For these reasons, the development of oocyte cryopreservation technologies in pigs was one step behind compared to other species. By the 1990s, it became clear that porcine oocytes do not tolerate traditional slow freezing methods (Didion et al., 1990). On the other hand, they apparently survive vitrification (reviewed by Zhou & Li, 2009). The first recorded attempt for porcine oocyte vitrification dates back to 1992 which resulted in approximately 24.5% survival of immature oocytes (Rubinsky et al., 1992). Blastocyst production from immature vitrified porcine oocytes was first reported by Fujihira et al. (2004) using intracytoplasmic sperm injection after in vitro maturation (IVM). Although significant improvements have been achieved in the vitrification of porcine oocytes during the following decades (Table 1), the production of the first piglets was reported only in 2014 by the transfer of in vitro produced blastocysts obtained from vitrified immature cumulus-oocyte complexes (COCs) (Somfai et al., 2014). Given that the optimum treatment with cryoprotective agents (CPAs) is used, the survival rate after vitrification/warming of cumulus-enclosed immature porcine oocytes at the germinal vesicle (GV) can exceed 90%, and over 10% of them can develop to the blastocyst stage after IVF (Table 1). This efficacy is nearly similar to those achieved in cattle immature oocytes (Hochi, 2022). However, developmental competence of vitrified immature oocytes to the blastocyst stage is still reduced compared with their non-vitrified counterparts (Somfai et al., 2014, 2015, 2023) which underlines the need for further improvements. On the other hand, resultant blastocysts appeared to be normal in terms of cell numbers (Somfai et al., 2023) and were able to develop to term (Somfai et al., 2014), which confirms that immature oocyte vitrification is a potent approach for female germplasm cryopreservation in pigs. The following chapters will discuss the advantages of immature oocyte vitrification at the GV-stage in pigs; the impact of the vitrification protocol on success; the effects of vitrification on subsequent development and the current status of practical application of immature oocyte vitrification for gene banking in pigs.
| Device | Equilibration | Vitrification | Oocyte Survivalb | % blastocyst after IVFc | Notes | References | |||
|---|---|---|---|---|---|---|---|---|---|
| Permeating CPA (% v/v) | Duration | Permeating CPA (% v/v) | Sugar (M) | Duration | |||||
| Slide | None → PG (17.5%) + GLY (2.5%) | 3 min | PG (17.5%) + GLY (5%) | Sucrose 0.05 | 6 min | No data | No data | 24.5% maturation (MI + MII) | Rubinsky et al. (1992) |
| Straw | EG (3% → 40%) | 13 min | EG (40%) | None | No data | No data | No data | 22% maturation. CB pre-treatment | Isachenko et al. (1998) |
| OPS | EG (5% → 30%) | 12.5 min | EG (40%) | None | 25 s | No data | No data | 6.1% maturation (MII) | Rojas et al. (2004) |
| Cryotop | EG (5% → 20%) | 11 min | EG (40%) | None | 1 min | No data | 13.5% | 37.1% maturation. CB pre-treatment. Embryos generated by ICSI | Fujihira et al. (2004) |
| Straw | EG (20%) | 10 min | EG (40%) | Sucrose 0.5 | 1 min | 47.5% | 0% | Partial delipation | Park et al. (2005) |
| SSV | EG (2%) + DMSO (2%) | 10-15 min | EG (17.5%) + DMSO (17.5%) | Sucrose 0.4 | <30 s | Appr. 75% | 9.5% | Gupta et al. (2007) | |
| Straw | EG (5% → 30%) | 9 min | EG (40%) | Sucrose 0.75 | 30 s | No data | No data | 20% maturation, CB pre-treatment | Huang et al. (2008) |
| Pipet tip | EG (7.5%) + DMSO (7.5%) | 3 min | EG (15%) + DMSO (15%) | Sucrose 0.5 | 1 min | Appr. 40% | 2.5% | Ruthenium red supplementation | Nakagawa et al. (2008) |
| SSV | EG (4%) | 15 min | EG (35%) | Trehalose 0.3 | 30 s | 27.7% | 5.1% | CB pre-treatment | Somfai et al. (2010) |
| Straw, SOPS Cryotop | EG (4%) | 15 min | EG (35%) | Trehalose 0.4 | 20 s | 4–17% | 3% | Fernández-Reyez et al. (2012) | |
| SSV | EG (2%) + PG (2%) | 15 min | EG (17.5%) + PG (17.5%) | Trehalose 0.3 | 30 s | 42.6% | 10.7% | CB pre-treatment | Somfai et al. (2013) |
| SSV | EG (2%) + PG (2%) | 15 min | EG (17.5%) + PG (17.5%) | Trehalose 0.3 | 30 s | 87.1% | 5.2% | CB pre-treatment warming at 42°C. Piglets obtained after ET. | Somfai et al. (2014) |
| Cryolock | EG (7.5%) + DMSO (7.5%) | 3 min | EG (16%) + DMSO (16%) | Sucrose 0.4 | <30 s | 96.0% | No data | Casillas et al. (2014) | |
| SOPS | EG (10%) + DMSO (10%) | 3 min | EG (20%) + DMSO (20%) | Sucrose 0.6 | 1 min | 67.0% | 6.6% | Nohalez et al. (2015) | |
| Cryolock | EG (7.5%) + DMSO (7.5%) | 3 min | EG (16%) + DMSO (16%) | Sucrose 0.4 | <30 s | No data | 16% | Granulosa co-culture | Casillas et al. (2015) |
| SSV | EG (2%) + PG (2%) | 13–15 min | EG (17.5%) + PG (17.5%) | Sucrose 0.3 | 30 s | 80.3% | 5.9% | CB pre-treatment. Warming at 42°C. | Somfai et al. (2015) |
| Cryotop | EG (2%) + PG (2%) | 13–15 min | EG (17.5%) + PG (17.5%) | Sucrose 0.3 | 30 s | 61.1% | 2.9% | CB pre-treatment. Warming at 42°C. | Santos et al. (2017) |
| Cryolock | EG (7.5%) + DMSO (7.5%) | 3 min | EG (16%) + DMSO (16%) | Sucrose 0.4 | <30 s | 66% | 14% | Casillas et al. (2018) | |
| MD | EG (2%) + PG (2%) | 13–15 min | EG (17.5%) + PG (17.5%) | Sucrose 0.3 | 30 s | 93.1% | 9.4% | Native miniature pig. | Somfai, Nguyen, et al. (2019) |
| OPS | EG (7.5%) + DMSO (7.5%) | 5 min | EG (15%) + DMSO (15%) | Sucrose 0.4 | 30 s | 78.0% | 3.3% | With cholesterol loaded cyclodextrin | Chen et al. (2019) |
| Cryoleaf | EG (2%) + PG (2%) | 13–15 min | EG (17.5%) + PG (17.5%) | Sucrose 0.3 | 30 s | 83.4% | 14.2% | Glycine and melatonin supplementation | Tang et al. (2022) |
| Cryotop | EG (2%) + PG (2%) | 13–15 min | EG (17.5%) + PG (17.5%) | Sucrose 0.3 | 30 s | 97.4% | 27.7% | Nguyen et al. (2022) | |
- a Data from reports available for the authors are presented in this table. Results acquired by parthenogenetic activation or intracytoplasmic sperm injection for embryo production (except from Fujihira et al., 2004) are not included.
- b Based on membrane integrity (morphology).
- c Based on total number of cultured oocytes.
- Abbreviations: CB, cytochalasin B; CPA, cryoprotectant agent; DMSO, dimethyl sulfoxide; EG, ethylene glycol; GLY, glycerol; IVF, in vitro fertilization; MD, microdrop; MI, metaphase I; MII, metaphase II; OPS, open pulled straw; PG, propylene glycol; SOPS, superfine open pulled straw; SSV, solid surface vitrification.
2 WHY PRESERVE IMMATURE OOCYTES?
In mammals, cryopreservation of oocytes at the immature stage is optional when access to matured oocytes is not possible such as in case of sexual inactivity (immature or off-season animals) and/or when tools/facilities to obtain matured oocytes (i.e., by in vitro maturation) are not given. In humans, cryopreservation of immature oocytes is an option to preserve germ cells from women whose reproductive ability is jeopardized by certain factors such as chemotherapy (Rodriguez-Wallberg & Oktay, 2010). Interestingly, in pigs, oocyte vitrification at the immature stage seems to have certain advantages as well (Figure 2).
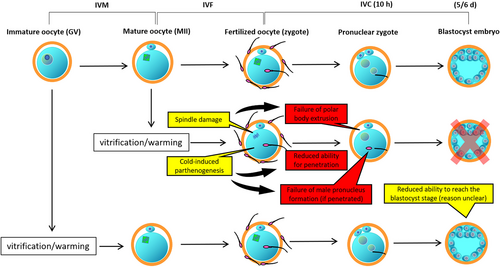
Early studies demonstrated that mammalian oocytes such as those of humans, cattle, and pigs are more resilient to certain cryopreservation protocols at the mature (metaphase II = MII) stage than at the immature (GV) stage (Goud et al., 2000; Otoi et al., 1995; Rojas et al., 2004). Hence, in human fertility clinics, vitrification of matured oocytes has become an accepted procedure (Rienzi et al., 2017), whereas vitrification of immature oocytes is still considered a challenge and is generally rejected as a reasonable approach (Son et al., 2019). For the same reasons, our team first focused on porcine oocyte vitrification at the mature stage. However, despite reasonable survival rates, the subsequent development of the vitrified oocytes after in vitro fertilization (IVF) was dramatically reduced and the embryos generated from vitrified MII stage oocytes failed to develop to the blastocyst stage (Somfai et al., 2007). On the other hand, when freshly collected immature oocytes were vitrified by the same method, the surviving oocytes had the ability to develop to blastocysts with high quality despite the low survival rates (Somfai et al., 2010). Direct comparison of GV and MII stage oocytes for vitrification confirmed, that despite the higher survival, mature oocyte vitrification was associated with failure of embryo development to the blastocyst stage after IVF (Egerszegi et al., 2013) unlike after GV-stage oocyte vitrification. The difference lies in the peculiar characteristics of the matured oocytes. In normal case, such oocyte remains temporarily arrested at the MII stage until the fertilizing sperm activates it causing the resumption of the meiotic process, the extrusion of the second polar body and eventually the formation of both male and female pronuclei. In matured oocytes, the MII stage is characterized by a functional spindle and properly arranged organelles (such as cortical granules and mitochondria) which are maintained by a delicate balance of cytoplasmic kinases (Coticchio et al., 2015; Gordo et al., 2001). These are all necessary to ensure normal fertilization and subsequent development. However, in porcine oocytes, vitrification at the MII stage generates at least three different kinds of alterations (Figure 2), which do not cause instant cell death (i.e membrane damage) but upset the cytoplasmic balance resulting in the failure of normal fertilization and development during subsequent IVF and embryo culture. These involve (1) the vitrification-induced parthenogenetic activation which results in the reduction of sperm penetration or (in penetrated oocytes) the failure of the male pronuclear formation and (2) the damage of the meiotic spindle which results in the failure of the second polar body extrusion (Egerszegi et al., 2013; Somfai et al., 2007). Furthermore, these are accompanied by (3) a compromised antioxidant defense system causing the accumulation of the reactive oxygen species (ROS) in cytoplasm (Somfai et al., 2007), which is also detrimental for embryo development. Extensive research has demonstrated that vitrification severely compromises mitochondrial activity in matured porcine oocytes which increases the intracellular level of ROS and triggers apoptosis (Dai et al., 2015, 2016; Niu et al., 2016; Vallorani et al., 2012). These stresses act upon MII stage oocytes simultaneously during vitrification and warming which explains why IVF of these oocytes is characterized with notoriously low embryo development rates despite high survival.
On the other hand, when fully grown cumulus-enclosed porcine oocytes are vitrified immediately after collection then subjected to IVM and IVF after warming, these problems do not arise. Nuclear and cytoplasmic maturation, fertilization events and cytoplasmic ROS levels appeared to be normal in surviving GV-stage oocytes (Appeltant et al., 2017; Santos et al., 2018; Somfai et al., 2010, 2014), and there were no signs of apoptosis in oocytes and subsequently developing embryos (Somfai et al., 2020). Oocyte vitrification before the formation of the spindle has been considered to be a reasonable way to prevent spindle damages (Eroglu et al., 1998). During the 44–48 h of subsequent IVM culture, vitrified porcine oocytes have a chance to undergo certain repair processes (detailed below in chapter “The competence of surviving oocytes, sublethal alterations and recovery”), which can potentially contribute to their better developmental competence to the blastocyst stage (Appeltant et al., 2017; Somfai et al., 2012). Nevertheless, it is important to point out that oocytes vitrified at the end of the GV stage cannot regain their ability to complete maturation (Somfai et al., 2012); therefore, vitrification within 2–3 h after collection is recommended (Santos et al., 2018). Furthermore, the main initial problem, i.e. the high frequency of membrane damage after the vitrification of GV-stage oocytes can be solved with the adjustment of CPA treatment and the warming temperature specifically for this meiotic stage (discussed in the next chapter). Taken together, the above mentioned indicate that, currently in pigs, vitrification of freshly collected cumulus-enclosed oocytes at the GV stage is more advantageous than vitrification at the MII stage. Interestingly, some reports on bovine oocytes came to the same conclusion (Yodrug et al., 2021; Zhou et al., 2010). This suggests the possibility that the observations and achievement made in porcine oocytes may be applied for oocyte vitrification in other species as well.
3 FACTORS AFFECTING THE SUCCESSFUL VITRIFICATION AND SUBSEQUENT UTILIZATION OF IMMATURE PORCINE OOCYTES
3.1 The vitrification method
To date, several vitrification methods have been applied for the preservation of immature porcine oocytes with very variable results (Table 1). Among these reports, there is little or no consistency in the exact vitrification protocol used. The phrase “vitrification method” may refer to either the device only or the device combined with the CPA treatment procedure. Therefore, the following sub-chapters address these points separately. Furthermore, it is important to point out, that some studies reported different results using the same vitrification protocol (Table 1) which indicates that factors other than the vitrification protocol (such as the skills of the technician) may also influence the results. It is worth to note that large numbers of porcine oocytes used for IVEP are usually obtained from slaughterhouse-sourced ovaries. Female pigs are commonly slaughtered at a market weight that is attained prior to reaching sexual maturity. It is known that the quality of oocytes from prepubertal gilts are inferior to those from mature sows (Grupen, 2014) This raises the question about the intrinsic quality of the oocytes used in vitrification studies which may explain some differences between studies. Other factors which may affect results (hence differences between studies) are season, follicle size and the IVM system (reviewed by Grupen, 2014).
3.1.1 Device
It is generally accepted that the vitrification device determines the cooling/warming rate during the process; hence it has been considered as major factor for the success for vitrification (Kuwayama, 2007). Among many devices, the Cryotop employing a minimum volume (less than 0.1 μl) of the vitrification solution was proven to provide a relatively high cooling rate (approximately 37,500 C/min) (Kuwayama, 2007; Sansinena et al., 2011). A study on matured porcine oocytes demonstrated the superiority of the Cryotop device compared with the Open Pulled Straw (OPS) method (Liu et al., 2008). However, using a different CPA treatment protocol, immature porcine oocytes could be effectively vitrified by the direct plunging of the oocytes into liquid nitrogen in 1- to 2-μl microdrops (MDs) of the vitrification solution with success rates similar to those achieved by the Cryotop device with minimum volume cooling (Appeltant et al., 2018). This suggests that when optimum CPA treatment is used, vitrification devices employing 2 μl or less of vitrification solution may be similarly effective. This suggestion is in line with the earlier results of Fernández-Reyez et al. (2012) and a similar conclusion was made on in vitro produced porcine blastocysts (Bartolac et al., 2015).
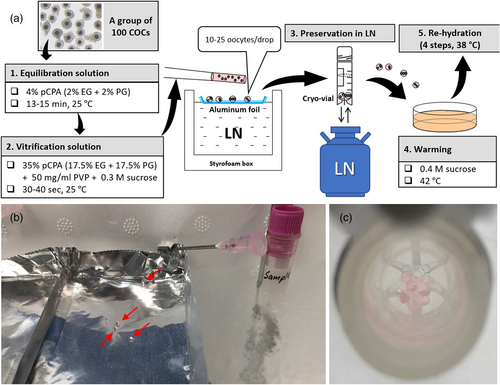
In respect of the above mentioned, the capacity and biosafety, availability and the price should determine which type of vitrification device is optimum for oocyte cryopreservation for each case (discussed in the last chapter). Many vitrification devices such as Cryotip, Cryoloop and Cryotop are available in closed version to prevent cross-contamination during storage (Castelló et al., 2018; Larman et al., 2014). However, they can accommodate only a limited number (3–10) of oocytes, hence they are not optimum when large numbers (over 100) of oocytes are to be vitrified at once. Vitrification of oocytes in MDs either in liquid nitrogen (LN) or on a cold metal surface (solid surface vitrification = SSV) is a deviceless approach which allows the fast cryopreservation of oocytes in groups of up to 100 in multiple MDs (Somfai & Kikuchi, 2021). Vitrified MDs can be stored in closed cryotubes (Figure 3). Such high-capacity vitrification method is advantageous in pigs which are multiparous (thus, several embryos must be transferred to one recipient to achieve pregnancy), and their IVEP is often characterized by limited embryo production rates (Grupen, 2014) (hence, high numbers of oocytes are required for each experiment). Therefore, the MD vitrification is clearly advantageous when oocytes are to be cryopreserved to conduct large scale research experiments. Nevertheless, this approach has a disadvantage that approximately 1%–5% of the oocytes are lost during preservation. Therefore, when only limited number of oocytes are available (e.g., gene banking for individual animals with low reproductive performance) other vitrification devices may offer a better choice.
3.1.2 The CPA treatment and warming protocols
For the vitrification of immature porcine oocytes, almost all of the previous studies (with one exception) (Table 1) employed a relatively high (30–40% v/v) total concentration of permeating CPAs (pCPAs) combined with a non-permeating sugar (0.3–0.75 M of trehalose or sucrose) in the final vitrification solution for a relatively short treatment period (20–60 s) before cooling. As for the permeating CPA, its permeability and toxicity for oocytes are known to affect the success of vitrification (Vajta & Kuwayama, 2006). The membrane of mammalian oocytes at the GV stage appears to have lower permeability to CPA than that at the MII stage (Agca et al., 1998; Le Gal et al., 1994). Consequently, the replacement of ethylene glycol (EG) with the more permeable propylene glycol (PG) at the same concentration (35% v/v) as a sole permeating CPA for vitrification dramatically increased the survival of immature oocytes (from 27.8% to 73.9%) without affecting maturation and fertilization (Somfai et al., 2013). However, its toxic effects severely compromised the subsequent embryo production (Somfai et al., 2013). The combination of different permeating CPAs is apparently more advantageous than using a single one at the same final concentration since it improves the permeation of each CPA (Vicente & Garcia-Ximenez, 1994) and reduces the specific toxic effects of certain CPA such as that of propylene glycol (Somfai et al., 2013). In our system, the combination of 17.5% EG and 17.5% PG resulted in reasonable survival rates without apparent toxic effects on embryo development (Somfai et al., 2013). Several studies (typically using OPS, superfine OPS, or Cryotop vitrification methods) applied the combination of EG and dimethyl sulfoxide (DMSO) as permeating CPAs for the vitrification of immature porcine oocytes (Table 1) according to the original protocols established in other species or cell types (Kuwayama, 2007; Vajta et al., 1997). For the vitrification of immature porcine oocytes, the combinations of EG + PG and EG + DMSO appeared to be equally effective in terms of subsequent embryo production (Nohalez et al., 2015; Somfai et al., 2015) although blastocysts obtained in the EG + DMSO group tended to have reduced cell numbers (Somfai et al., 2015). However, some reports suggest that the advantages of pCPA combinations may depend on the actual vitrification protocol (Nohalez et al., 2015; Wu et al., 2017).
In several previous studies (including many of ours), pre-treatment with cytochalasin B was frequently used to improve the flexibility of the oocyte membrane and thus its resistance to damages during vitrification and warming (Table 1). Nevertheless, later Appeltant et al. (2018) demonstrated that cytochalasin B pre-treatment had no positive effect whatsoever (at least in our vitrification system), therefore this reagent can be omitted from the system.
As for the non-permeating CPA during vitrification sucrose and trehalose has been proven equally effective (Somfai et al., 2015) therefore their availability and cost may determine the preference of choice.
A key factor for the outcome of vitrification is the equilibration treatment with pCPA before vitrification. Many studies applied a relatively high (15% v/v) total pCPA concentration for a short time (typically 7.5% EG + 7.5% DMSO for 3 or 5 min), following the original protocols for OPS and Cryotop (Table 1). Others (including our group) applied a lower total concentration (4%) of pCPA for a longer period (13–15 min) based on the previous results of Papis et al. (2000) on bovine oocytes. When directly compared, the latter approach was found to be superior for the vitrification of immature porcine oocytes (Somfai et al., 2015). Hence, our method of choice for CPA treatment during immature oocyte vitrification is equilibration in a combination 2% EG + 2% PG for 13–15 min followed by a very short (30–40) treatment in 17.5% EG + 17.5% PG + 0.3 M sucrose + 50 mg/ml polyvinylpyrrolidone (PVP) (Somfai et al., 2015). This CPA treatment has no apparent toxic effects on the maturation, fertilization and subsequent embryo development of oocytes (Somfai et al., 2013). It must be noted that equilibration treatment at room temperature rather than at 38–37°C appears to be beneficial for the subsequent embryo development of vitrified immature oocytes (Appeltant et al., 2018; Wu et al., 2017). Using this CPA treatment, the survival rate of immature porcine oocytes after MD vitrification could be increased to 87.1% by adjusting the temperature during warming of vitrified samples to 42°C, which prevented the reduction of warming medium temperature below 35°C during consecutive insertion of six vitrified MDs (Somfai et al., 2014). Based on the above mentioned, we set up a CPA-treatment and warming protocol optimized specifically for the MD vitrification of immature porcine oocytes (Figure 3). To date, the highest survival and subsequent blastocyst production rates by IVM/IVF of immature cryopreserved porcine oocytes (97.4% and 27.7%, respectively) was achieved using this exact CPA treatment and warming protocol but with Cryotop sheets as vitrification device (Nguyen et al., 2022).
3.2 The presence of cumulus cells
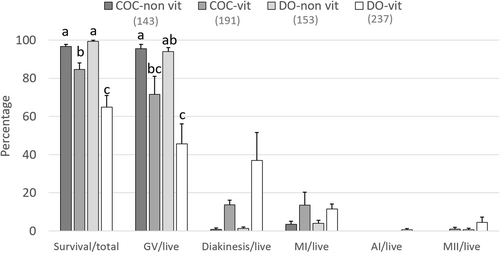
In previous studies, it has been suggested that the presence of cumulus cells on the zona pellucida might reduce the success of immature oocyte vitrification due to blocking/slowing CPA permeation (Casillas et al., 2014, 2015). Therefore, these authors denuded the oocytes before vitrification. This approach requires a co-culture with granulosa cells for efficient embryo production (Casillas et al., 2014, 2015). On the other hand, cumulus cells are known to be important during IVM for the cytoplasmic maturation of porcine oocytes by maintaining intracellular glutathione at a level necessary to support embryo development (Maedomari et al., 2007). Also, cumulus cells regulate the meiotic resumption of oocytes (reviewed by Appeltant et al., 2016). For this reason, our group fundamentally refrains from oocyte denudation before vitrification. In a recent study, we compared the survival after vitrification/warming of cumulus-intact and denuded immature porcine oocytes in our system and revealed that denudation before vitrification significantly reduced the percentage of membrane intact oocytes after warming (Nguyen et al., 2021; Figure 4). Therefore, we concluded that the presence of an intact cumulus compartment is in fact beneficial for oocyte survival during vitrification/warming. A similar conclusion was made in an earlier report using horse oocytes (Tharasanit et al., 2009). An interesting approach was reported by Jia et al. (2019) who co-cultured vitrified COCs with non-vitrified ones which improved the developmental competence of vitrified oocytes, suggesting the possibility that paracrine factors excreted by healthy COCs might be beneficial for the recovery of vitrified oocytes from non-lethal damages.
3.3 The lipid content of oocytes
In the early studies by Nagashima et al. (1994, 1995), it was demonstrated that the high intracellular lipid content of porcine oocytes and resultant embryos is responsible for their sensitivity to low temperatures which hinders traditional slow freezing. Mechanical lipid removal indeed dramatically improved the freezing tolerance of early porcine embryos (Nagashima et al., 1995). However, adoption of this technique for the vitrification of immature oocytes resulted in moderate success (Park et al., 2005). The negative aspects of this technique are that (1) it requires the removal for a certain amount of cumulus cells (which itself may hinder development potential) and also that (2) it comprises the integrity of zona pellucida which does not meet the international standards of sanitary handling (Stringfellow, 1998). Interestingly, our research revealed a significant difference in the lipid content of the oocytes of native Vietnamese Ban pigs and those of a western cross breed (Landrace x Large White) (Somfai, Nguyen, et al., 2019). However, when we applied our vitrification protocol on oocytes of these breeds, the survival and development rates were not significantly different (Somfai, Nguyen, et al., 2019). This suggests that, when optimum vitrification protocol is used, the lipid content of the immature oocyte may not be a decisive factor for the success of vitrification.
3.4 Post-warming culture (IVM)
Previous studies revealed that vitrification at the GV stage can induce several different types of intracellular damages and alterations in porcine oocytes which can potentially reduce subsequent embryo development. Some examples are the fragmentation of the nucleolus, the damage of microfilaments, premature nuclear progression, and the damage of mitochondria (discussed in the following chapters). Interestingly, oocytes seem to be able to recover from some of these alterations at least to some extent during their post-vitrification IVM culture, such as in case of nucleolus, microfilament and mitochondrial damage (Appeltant et al., 2017; Ito et al., 2020). Although the exact mechanisms of the nucleolus and microfilament recovery in oocytes are not clear, it can be assumed that the composition of the medium (i.e., the presence or lack of necessary factors) may affect these processes. For instance, the recovery from vitrification-related mitochondrial damage and thus subsequent embryo development in immature porcine oocytes could be enhanced by medium supplementation with resveratrol during post-warming IVM culture (Ito et al., 2020). Furthermore, the reagents used for meiosis synchronization during IVM have a major effect on the efficacy of subsequent in vitro embryo production from vitrified oocytes (Nguyen et al., 2022). These examples demonstrate that the medium composition during post-warming culture can indeed affect the developmental competence of vitrified oocytes and that the enhancement of recovery processes is a possible way to improve embryo production rates.
4 THE COMPETENCE OF SURVIVING OOCYTES, SUBLETHAL ALTERATIONS AND RECOVERY
In many studies, high percentages of oocyte survival were achieved after vitrification of immature porcine COCs (Table 1). Also, in several studies we demonstrated, that COC vitrification at the GV stage does not reduce the ability of surviving oocytes to undergo nuclear maturation and fertilization (Nguyen et al., 2022; Somfai et al., 2010, 2013, 2014, 2015). However, in all studies, the competence of vitrified oocytes to develop to the blastocyst stage was lower than that of the non-vitrified control. This suggests that vitrification at the GV stage induces damages or structural alterations in surviving oocytes which are manifested later, during embryo development. To date, several different types of cellular alterations have been described in vitrified porcine oocytes and their exact mechanisms and/or contribution to the reduced developmental competence is yet to be clarified. For the further improvement of vitrification and culture protocols, these phenomena should be considered, clarified, and addressed. In the following chapters, some of the alterations which have been observed in vitrified immature porcine oocytes are discussed.
4.1 The fragmentation of the nucleolus
Phase contrast microscopy revealed that the CPA treatment during the vitrification process causes the fragmentation of the nucleolus precursor body (NBP) in the GV in approximately 50% of the surviving immature oocytes whereas none of the 125 control oocytes showed such phenomenon (Appeltant et al., 2017). On the other hand, the ratio of the oocytes having multiple NPBs instead of a single NPB in the GV was significantly reduced after subsequent IVM for 20 h in both the vitrified and CPA groups, suggesting the recovery from NPB fragmentation. Neither the mechanisms of recovery, not the impact of NPB fragmentation, on subsequent development has been clarified to date. Nevertheless, it is noteworthy that the CPA treatment of our vitrification protocol does not reduce the competence of vitrified GV stage oocytes to develop to the blastocyst stage (Somfai et al., 2013), which suggests that NPB fragmentation may not be a major factor responsible for reducing the competence of vitrified oocytes.
4.2 The damage of the cytoskeleton and transzonal projections
The alteration of actin microfilaments in vitrified immature porcine oocytes was reported firstly by Rojas et al. (2004). In their report, both CPA treatment (EG) and OPS vitrification dramatically reduced the percentage of oocytes with normal actin microfilaments which was associated with the complete failure of cleavage after IVF (Rojas et al., 2004). Nevertheless, it is not clear if the investigated oocytes were alive or dead (i.e., membrane damaged) since selection of live oocytes for assay is not described in the study. It is noteworthy that, in our studies, vitrification of immature pig oocytes in solely EG-based media was associated with low survival rates (Somfai et al., 2010, 2013). Later, using a similar vitrification protocol, Wu et al. (2006) confirmed the alterations of cortical microfilaments in surviving oocytes. Since we use a completely different vitrification protocol employing a CPA treatment which itself does not reduce subsequent development, our group also investigated the effects of vitrification and CPA treatment on microfilaments in immature COCs. We have confirmed that vitrification but not CPA treatment caused the deformation of cortical microfilaments of the surviving oocytes which was manifested by the formation of clusters of actin under the oolemma immediately after warming (Appeltant et al., 2017). Interestingly, these clusters completely disappeared within 20 h of subsequent IVM culture suggesting a recovery mechanism for vitrification-related malformations of filamentous actin (F-actin). As a result, at 44 h IVM, the morphology of actin microfilaments did not differ between the vitrified and non-vitrified oocytes (Appeltant et al., 2017) which corroborates our previous results (Egerszegi et al., 2013). On the other hand, another study reported abnormal actin microfilaments in early embryos obtained from porcine oocytes vitrified at the GV stage (López et al., 2021), suggesting that repair mechanisms can be compromised. However, this study applied a different vitrification protocol and the effect of CPA treatment on the results remained unclear. Further research is necessary to clarify the contribution of F-actin alterations to the compromised developmental competence of vitrified oocytes and the impact of the CPA protocol.
In our previous study (Appeltant et al., 2017), visualization of filamentous actin by confocal microscopy allowed the study of transzonal projections (TZPs) within the zona pellucida which are known to play a pivotal role in gap junctional communication between the oocyte and the cumulus cells (Clarke, 2022). We found that vitrification itself but not CPA treatment of COCs significantly reduced the number of TZPs by approximately 30% and the difference between the vitrified and non-vitrified oocytes could be detected even after 20 h of subsequent IVM culture (Appeltant et al., 2017). Gap junctional communication through TZPs between the oocyte and the cumulus cells is important for the control of meiotic resumption and the cytoplasmic maturation of porcine oocytes (Appeltant et al., 2016; Mori et al., 2000) and cumulus expansion during IVM (Appeltant et al., 2015). Nevertheless, in vitrified COCs, the cytoplasmic maturity of oocytes (measured by glutathione and ATP contents) and the extent of cumulus expansion was similar to those of the non-vitrified control (Appeltant et al., 2017). This indicates that the reduction of TZP abundance by 20%–30% was not extensive enough to affect these features. On the other hand, vitrification but not CPA treatment was found to trigger premature nuclear resumption of oocytes (Appeltant et al., 2017) (discussed in the next chapter) which could theoretically be related to TZP damage.
4.3 Premature meiotic resumption
In porcine oocytes, cAMP-dependent protein kinase (PKA) maintains meiotic arrest at the GV stage during the first 10–20 h of IVM and cumulus cells play an important role in this process through jap junctional communication (GJC) (Appeltant et al., 2016). However, when immature COCs were vitrified by our method, approximately 20% of the oocytes resumed meiosis prematurely even in the presence of 1-mM dibutyryl-cAMP (dbcAMP), which is a membrane permeable cAMP analogue used to block premature meiosis during the first half of IVM in order to synchronize subsequent maturation (Appeltant et al., 2017). Since vitrification also compromised the integrity of TZPs, it seemed plausible that a reduction in GJC might be responsible for the vitrification-related premature meiosis. To clarify if gap junctional damage can lead to premature meiosis, we mechanically removed all cumulus cells from some freshly collected COCs with a fine glass capillary eliminating GJC, whereas other oocytes were processed as intact COCs. Then, in both groups, some oocytes were vitrified/warmed, whereas others were processed without vitrification. Then all oocytes were subjected to IVM culture in the presence of 1-mM dbcAMP for 22 h. The meiotic status of denuded oocytes (DOs) without or after vitrification were compared by orcein staining with those of intact COCs without or after vitrification. Interestingly, vitrification triggered premature meiosis resumption in approximately 20% of oocytes in both vitrified COCs and DOs in a similar manner whereas in both non-vitrified COCs and DOs dbcAMP could maintain the GV stage with similar efficiency (Figure 4). This demonstrates that in the presence of dbcAMP, the total damage of gap junctions itself in the group without vitrification cannot trigger premature meiotic resumption of oocytes and that vitrification-related premature meiosis occurs irrespective of the presence/absence of cumulus cells and the damage of gap junctions. Nevertheless, the mechanism of vitrification-related premature meiosis remains to be clarified.
The impact of vitrification-related premature nuclear progression on embryo development is also a matter of question. During IVM, premature meiosis of some oocytes is known to cause heterogeneity in cytoplasmic maturity and aging status in the population of cultured oocytes which reduces the efficacy of in vitro embryo production. For this reason, synchronization of oocyte maturation by the temporal inhibition of meiosis at the prophase (i.e. the GV stage) during the first 20–24 h of IVM has become a common practice in pigs (Grupen, 2014). In porcine oocytes, it can be achieved either by maintaining high intracellular cAMP levels (Appeltant et al., 2016) or by the inhibition of the metaphase promoting factor (MPF) (Motlik et al., 1998). The former approach can be achieved using 1-mM dbcAMP (Funahashi et al., 1997), whereas the latter way is achieved effectively using reversible inhibitors of p34cdc2 protein kinase (the catalytic subunit of MPF) such as roscovitine (Marchal et al., 2001) which applied at 25 μM was reportedly beneficial for fresh porcine oocytes (Zhu et al., 2020). We compared these two approaches for the nuclear synchronization of vitrified immature oocytes and found that dbcAMP could impede but not completely prevent premature meiosis in vitrified oocytes whereas roscovitine was more efficient. However, for subsequent embryo production, the use of roscovitine was adverse whereas dbcAMP was beneficial (Nguyen et al., 2022). Since the problem of vitrification-related premature meiosis could not be resolved yet, further research will be needed to address this point. Also, it remains to be elucidated whether the vitrification -related premature meiosis is the cause of reduced development or only an indicator of another problem in surviving oocytes. For instance, meiotic resumption is known to require intracellular Ca2+ ([Ca2+]i) oscillations in oocytes (He et al., 1997). [Ca2+]i release from intracellular stores might initiate meiotic resumption. The Ca2+ homeostasis in oocytes is controlled by cytoplasmic organelles such as the endoplasmic reticulum (ER) and mitochondria which store Ca2+ (Dumollard et al., 2007; Wakai et al., 2019). Although the effects of vitrification on the ER of porcine immature oocytes remain to be elucidated, it reportedly exerts stress on the ER in matured mouse oocytes (Zhao et al., 2015). Mitochondria are also known to be damaged by vitrification (discussed in the next chapter). It was demonstrated in bovine matured oocytes that vitrification induces the release of Ca2+ from intracellular stores into the cytoplasm (Wang et al., 2017). In this respect, premature nuclear progression may be an indicator of damage of Ca2+ regulation system. Further research will be necessary to verify this theory.
4.4 Mitochondrial damage
In mammalian oocytes, mitochondria play pivotal roles in energy production, Ca2+ homeostasis, signaling during fertilization, ROS regulation, and apoptosis (Dumollard et al., 2007). Previous studies demonstrated that, in matured porcine oocytes, vitrification may alter the mitochondrial properties which contributed to reduced developmental competence via the activation of the apoptotic cascade (Dai et al., 2015, 2016). However, in immature oocytes, this may not be the case since vitrification by our method at the GV stage did not trigger apoptosis in porcine oocytes and resultant embryos (Somfai et al., 2020). Furthermore, similar levels of cytoplasmic glutathione, ATP, and hydrogen peroxide in vitrified and non-vitrified immature porcine oocytes during subsequent IVM (Appeltant et al., 2017; Santos et al., 2018; Somfai et al., 2010) suggested that mitochondrial activities may not be dramatically altered by vitrification. Nevertheless, there is evidence that mitochondrial damage occurs to some extent in the vitrified immature oocytes as well. Nguyen et al. (2023) demonstrated reduced mitochondrial activity in vitrified immature porcine oocytes. Although it appeared to be normalized during subsequent IVM, mitochondrial damage contributed to the reduced developmental competence since it could be mitigated by pretreatment with cyclosporine A and docetaxel resulting in improved embryo development (Nguyen et al., 2023). In an earlier study, supplementation of the IVM medium with resveratrol, known as an antioxidant, improved the developmental competence of oocytes irrespective of cytoplasmic ROS levels and apoptosis (Santos et al., 2018). Later, Ito et al. (2020) demonstrated that resveratrol acts through the activation of sirtuin 1, which induces mitochondrial synthesis facilitating the recovery of vitrified-warmed oocytes. These examples demonstrate that, in immature porcine oocytes, the recovery from certain vitrification-related non-lethal damages can be assisted during post-warming IVM culture.
4.5 DNA damage
Although several previous studies verified the detrimental effect of cryopreservation on the integrity of DNA in matured mammalian oocytes (for a review see Kopeika et al., 2015), this issue was not explored in porcine oocytes until recently. Also, studies on DNA damage that occurs during oocyte vitrification at the GV stage in any species are lacking. In early porcine embryos, increased levels of DNA double-strand breaks (DSBs) are associated with delayed early development and a compromised ability to reach the blastocyst stage (Bohrer et al., 2015). Recently we demonstrated that vitrification but not CPA treatment generates DSBs in the DNA of porcine oocytes at the GV stage which are transmitted to the subsequently developing cleavage stage embryos during IVM, IVF and embryo culture (Somfai et al., 2023). This is associated with a delay of early development and compromised capacity to reach the blastocyst stage. However, it does not seem to affect the quality of resultant blastocysts in terms of cell numbers and the frequency of apoptosis. These results suggest that vitrification-induced DNA damage is a major factor that contributes to the compromised development of vitrified GV-stage oocytes. Furthermore, vitrification of oocytes at the GV stage was found to activate the expression of the RAD51 gene, which is responsible for DNA damage-repair by homologous recombination (Somfai et al., 2023). This suggests the possible importance of DNA damage-repair by homologous recombination during post-warming culture in the vitrified oocytes for their development to the blastocyst stage. Further research will be necessary to clarify mechanisms and regulation of DNA damage-repair in vitrified oocytes and subsequently developing embryos.
4.6 Epigenetic alterations
During early development of mammalian embryos, epigenetic regulation of gene expression is crucial for the normality of subsequent fetal and even postnatal development (reviewed by Sciorio et al., 2023). Cryopreservation was found to induce various types of epigenetic modifications in mammalian cells including oocytes (Chatterjee et al., 2017). Also, oocyte vitrification was found to cause abnormal expression of certain genes in the organs of mice at the adult age (Zhang et al., 2022) and alter physiological indexes such as blood pressure and triglyceride level at the first filial generation (Huo et al., 2020). These raise concerns for the health of children conceived at human fertility clinics where vitrification has been accepted as a standard procedure for oocyte cryopreservation (Sciorio et al., 2023). In humans, ethical restrictions underline the importance of animal models for the research of this matter. In mice and cattle, vitrification of matured oocytes was reported to reduce the levels of global DNA methylation and to increase histone acetylation even in subsequent embryos which were associated with reduced blastocyst development (Chen et al., 2016; Liang et al., 2012; Yodrug et al., 2021). This suggests the contribution of altered epigenetic features to developmental failure. When porcine immature oocytes were vitrified in our system, reduced levels of global DNA methylation were detected in the oocytes after IVM which was apparently caused by the CPA treatment (Somfai et al., 2021). In another report, however, vitrification of mouse oocytes at the GV stage did not induce alterations of global DNA methylation measured by the intensity of 5-methylcytosine immunostaining after subsequent IVM (Yan et al., 2014). The differences in results between the two studies may be species-specific since the CPA used for vitrification and the method of DNA methylation analysis were similar. Interestingly, after porcine oocyte vitrification, the DNA methylation levels were restored during early embryo development (Somfai et al., 2021). The DNA methylation levels were similarly affected in the vitrified and CPA-treated groups; however, the latter showed no reduction in the percentage of blastocyst development whereas the former showed reduced blastocyst development. This suggests that, in the current system, CPA-related reduction in DNA methylation levels may not be responsible for the compromised ability of vitrified oocytes to develop to blastocysts. Nevertheless, its effect on fetal and postnatal development remains to be explored. Furthermore, vitrification was reported to alter histone acetylation and methylation in matured porcine oocytes (Spinaci et al., 2012). However, in immature oocytes the effects of vitrification on histone acetylation and methylation status and its impact on subsequent development is yet to be investigated.
5 PRACTICAL APPLICATIONS
As mentioned in the first chapter, in vitro preservation of precious and sensitive porcine genetic resources is an important task under the threat of epidemic diseases. Although oocyte cryopreservation is an obvious option for the preservation of female germplasm, in pigs it has not become widely applied. Until recently, only a single attempt to preserve oocytes in an indigenous pig breed (Mangalica) was reported (Varga et al., 2008). This is largely due to the delay in the development of assisted reproduction and oocyte vitrification technologies in pigs. Nevertheless, recent improvements in these technologies can now facilitate the application of oocyte vitrification at a practical level for gene banking. Vietnam has many native pig breeds with limited distribution area, and many of them are on the verge of distinction (Ba et al., 2020). In Japan, Agu is the only native pig breed, and its range of distribution is limited to Okinawa prefecture (Touma et al., 2019). Vietnamese and Japanese native pigs are highly popular as local delicacies; therefore, they represent a high-economic value (Le et al., 2016; Touma & Oyadomari, 2020), but they are currently threatened by ASF and Classical swine fever, respectively, which justifies their gene banking. For practical applications of cryopreservation protocols, the possibility of pathogen-transfer during processing and storage has to be considered (Bielanski, 2012). Therefore, closed vitrification system and defined media are recommended. Since vitrified microdrops can be preserved in closed cryotubes under LN and defined media were applied in the system (Appeltant et al., 2018), we used our MD vitrification protocol to cryopreserve oocytes of indigenous Vietnamese pig breeds and Agu pigs for gene banking. Vitrification of the immature oocytes of the Vietnamese Ban miniature pigs resulted in high survival and reasonable blastocyst development rates after in vitro fertilization (Somfai, Nguyen, et al., 2019) comparable to our previous results achieved in modern pig breeds. Also, we introduced our vitrification protocol for the gene banking of oocytes of Agu pigs in Okinawa. When oocytes were collected from Agu sows culled at 1.7–8.6 years of age on arbitrary days of estrus, 68.2% of the oocytes survived vitrification. The age of sows and the stage of estrus cycle had no effect on oocyte survival (Somfai, Oyadomari, et al., 2019). Although the results are promising, live offspring from vitrified Ban and Agu oocytes have not been produced to date. Oocyte vitrification for gene banking of other Vietnamese pig breeds and the Japanese Agu is currently in progress.
ACKNOWLEDGMENTS
The author was awarded the 2023 Society Award of the Japanese Society of Animal Science for his research on the improvement of vitrification preservation and efficient utilization of porcine oocytes. The author is grateful to Drs Takashi Nagai and Kazuhiro Kikuchi for the continuous support during the years, to Drs Elisa Santos, Ruth Appeltant, and Hiep Thi Nguyen for contributing to our work in porcine oocyte vitrification and to Ms. Mitsuru Nagai for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (21K05912).
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interest.




