Effect of different inflammation states on the antimicrobial components in milk of goat udders after milking cessation
Abstract
The aim of this study was to examine the effects of milking cessation under different inflammatory conditions on the changes in antimicrobial components in milk and the process of mammary gland involution. Twenty udder halves were divided into two groups: those with (LPS) and without (control) lipopolysaccharide infusion, followed by cessation of milking for 8 weeks. Milk samples were collected weekly. Udder tissue was collected 4 weeks after milking cessation to measure the area of the lobule and connective tissue. After milking cessation, the somatic cell count (SCC) in the control group increased, whereas that in the LPS group did not. Lactoferrin (LF) and cathelicidin (Cath)-2 concentrations increased in both groups, whereas only LF was significantly lower in the LPS group than in the control group at week 4. The Cath-7 and S100A8 concentrations were significantly lower in the LPS group than in the control group. The lobule area was higher, and the connective tissue area was lower in the LPS group than in the control group. These results indicate that inflammation at milking cessation decreased the concentrations of some antimicrobial components and interfered with mammary gland involution. Therefore, animals with mastitis should recover prior to the onset of the dry period.
1 INTRODUCTION
Mastitis is an inflammation of the udder caused by pathogenic infection. Mastitis frequently occurs during the dry period and early milking stages in dairy cows (Eberhart, 1986). Patients in the early and late dry periods have the highest risk of developing mastitis. The rate of new infections at the beginning of the dry period was reported to be approximately six to seven times higher than that observed during lactation, although the rate of new infections during the remaining dry period was very low (Bradley & Green, 2000; Larry Smith et al., 1985). Intramammary infections that occur during involution increase the risk of clinical mastitis during lactation.
The dairy industry typically has a dry period of approximately 40–60 days between late lactation and calving (Valizaheh et al., 2008). This period is critical for maintaining milk production and quality for the next lactation period and is essential from a cow health and animal welfare perspective (Bachman & Schairer, 2003). Mammary glands undergo contraction and tissue renewal during the dry period (Capuco & Akers, 1999). The sudden cessation of milking results in hyperemia of the mammary ducts and mammary epithelial cells, leading to increased intramammary pressure, which triggers mammary involution. These processes involve the apoptosis of various cell types (Quarrie et al., 1996). Regression and reconstruction of the mammary glands do not proceed normally under inflammatory conditions (Green et al., 2002). Therefore, preventing infections during dry periods is important.
Immune function is important for protecting the mammary glands from infection. Innate immunity, as well as established immunity, is required for the defense against mammary gland infections (Isobe, 2017). Antimicrobial components have direct antimicrobial activity against various microorganisms, such as viruses, bacteria, and fungi (Schauber & Gallo, 2008). Antimicrobial components such as lactoferrin (LF; Groves et al., 1965), cathelicidin (Smolenski et al., 2011), and S100 calcium-binding protein (Connor et al., 2008; Smolenski et al., 2015) are produced by the leukocytes and epithelial cells of the mammary gland. The concentrations of these antimicrobial components are increased in mastitic milk (Isobe, 2017). However, even during the dry period, LF concentration rises dramatically in the mammary gland after the cessation of milking (Nickerson, 1989; Sordillo et al., 1987), indicating that LF plays a role in the defense of the mammary glands during the dry period. However, it is unclear whether other antimicrobial components, such as LF, also change and whether inflammatory conditions affect their concentrations. Therefore, the purpose of this study was to elucidate changes in the milk concentration of antimicrobial components in mammary glands with and without inflammation after milking cessation.
2 MATERIALS AND METHODS
2.1 Experimental animals
Twenty udder halves of 12 non-pregnant Tokara goats in the late lactation period (body weight 22–54 kg; parity 1–4; milk yield <15 mL/day) were used in this study. The goats were fed 0.6 kg of hay and 0.2 kg of barley at 09:00 and 15:00 daily. Water and trace-mineralized salt blocks were freely accessible. This study was approved and performed in accordance with the guidelines of the Hiroshima University Animal Research Committee (no. C19–4).
2.2 Experimental design and sample collection
Goats were divided into lipopolysaccharide (LPS) (n = 10) and control (n = 10) groups. In the LPS group, mammary glands were infused with LPS (from E. coli O111:B4; Wako Pure Chemical Industries, Osaka, Japan) at a dose of 1 μg/5 mL in phosphate-buffered saline (PBS) after the last milking to create an inflammatory state. The day of the LPS infusion was considered week 0. LPS was not administered to the control group. Milking was stopped, and milk was collected weekly for 8 weeks. The deep area of the mammary tissue was collected at 4 weeks after the cessation of milking in both groups (n = 3 each) by biopsy. All biopsy instruments were autoclaved before use, and biopsy was conducted under deep sedation and anesthesia by intravenous injection of xylazine (Bayer HealthCare, Osaka, Japan) and pentobarbital (Somnopentyl; Kyoritsu Seiyaku Corporation, Tokyo, Japan). Mammary tissues were immediately fixed in 10% formalin, dehydrated by conventional methods, and embedded in paraffin (Purba et al., 2020).
2.3 Somatic cell count (SCC) measurement and milk viscosity/color
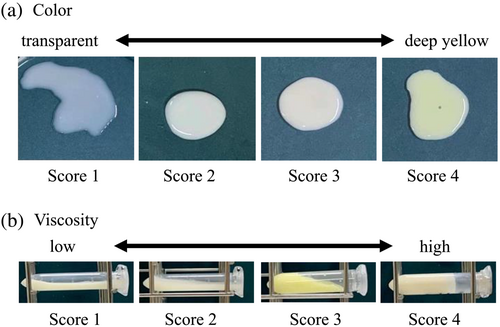
The viscosity of the milk was scored in four stages (Figure 1), ranging from watery and fluid (score 1) to hard starch syrup that did not flow even when the subject fell (score 4). The color of the secretions was judged visually and scored in four stages (Figure 1), from transparent (score 1) to deep yellow (score 4). Milk was centrifuged at 2300×g for 5 min at 4°C. Milk fat was removed, and the skim milk was stored at −30°C for ELISA. The precipitate was resuspended in PBS, and SCC was measured using a Countess Automated Cell Counter (Life Technologies Japan Ltd., Tokyo, Japan).
2.4 ELISA
The concentrations of LF, S100A7, S100A8, cathelicidin (Cath)-2, and Cath-7 were measured using competitive ELISA, as reported previously (Isobe et al., 2020; Purba et al., 2019; Zhang et al., 2014). The samples were diluted 5000, 2000, 1000, 20, and 50 times for LF, S100A7, S100A8, Cath-2, and Cath-7 determination, respectively. The intra- and inter-assay coefficients of variation were 6.5% and 9.3% for LF, 7.5% and 19.6% for S100A7, 9.1% and 11.3% for S100A8, 11.3% and 17.9% for Cath-2, and 3.7% and 20.0% for Cath-7.
2.5 Tissue staining
Hematoxylin and eosin (H&E) staining of paraffin sections was performed to visualize and quantify lobules and connective tissue areas. Five fields were randomly captured per half of the tissue section using an Eclipse E400 microscope and a Digital Sight DS-Fi1 camera (Nikon, Tokyo, Japan). Connective tissue and lobule areas were quantified using ImageJ software, as previously reported (Schneider et al., 2012).
2.6 Statistical analyses
Statistical analyses were performed using JMP Pro 16 software (SAS Institute Inc.), and the results were reported as least squares means (LSMs) and standard errors of LSMs (SEM). All data were checked for normality using the Kolmogorov–Smirnov test. The concentrations of LF, Cath-2, Cath-7, S100A7, S100A8, and SCC were log-transformed (Log10) and assessed using two-way ANOVA followed by Tukey's multiplex test. Differences were considered statistically significant at P < 0.05.
3 RESULTS
SCC was affected by the group and the interaction between group and time (P < 0.01; Figure 2a). Before milking cessation, the LPS group had a higher SCC than the control group, whereas the control group had a higher SCC at week 2. Furthermore, SCC in the LPS group was significantly lower than that in the control group (P < 0.05) at weeks 2, 3, 4, and 6.
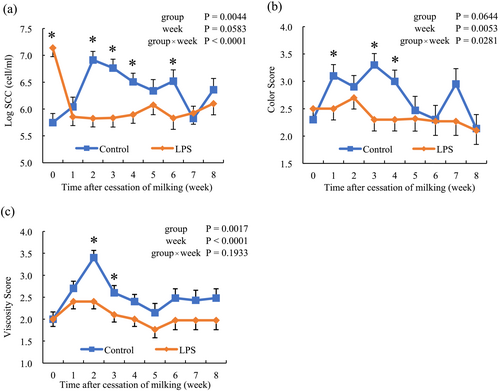
The state of milk was examined in terms of both color and viscosity, as shown in Figure 2. The milk color score was affected by the group and the interaction between the group and time (P < 0.01). After the cessation of milking, the color changed to yellow in the control group but not in the LPS group. At weeks 1, 3, and 4, the color score was significantly lower in the LPS group than that in the control group (P < 0.05). The milk viscosity score increased after cessation of milking in the control group, followed by a return to the basal level. No changes in milk viscosity were observed in the LPS group.
LF concentration increased after the cessation of milking in both groups and remained high. The LF concentration in the LPS group was significantly lower than that in the control group at week 4 (P < 0.05; Figure 3e).
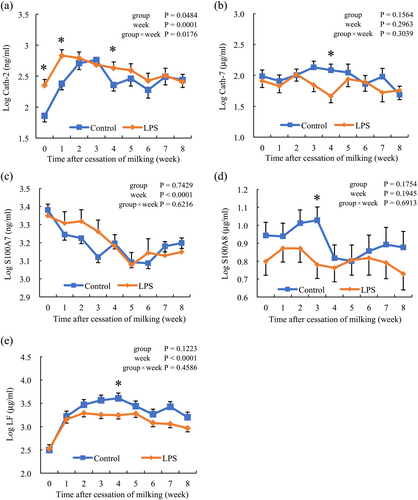
The Cath-2 concentration increased in both groups after the cessation of milking and was significantly higher in the LPS group than in the control group at weeks 0, 1, and 4 (P < 0.05; Figure 3a). Cath-7 concentration was not affected by group, time, or the interaction between group and time; however, at week 4, the LPS group had significantly lower levels than the control group (P < 0.05; Figure 3b). The S100A7 concentration decreased in both groups after the cessation of milking, and no difference was observed between the groups (Figure 3c). S100A8 concentration was not affected by group, time, or the interaction between group and time but was significantly lower in the LPS group than in the control group at week 3 (P < 0.05; Figure 3d).
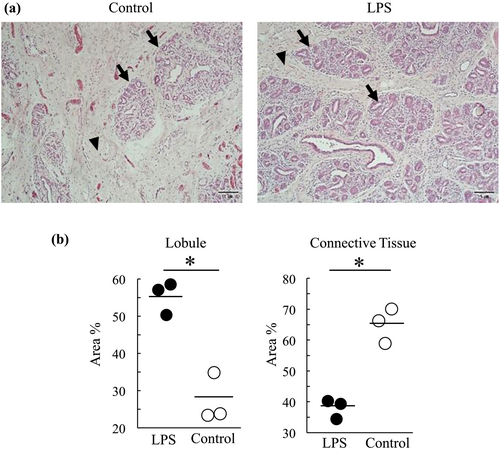
H&E staining was performed to quantify the mammary tissue microstructure, including the lobule and connective tissue areas, at week 4 (Figure 4a). The LPS group showed a higher percentage of lobular areas than the control group. Conversely, the LPS group showed a lower percentage of connective tissue in the total tissue area than the control group (P < 0.05; Figure 4b).
4 DISCUSSION
Mastitis frequently occurs immediately after dry-off, followed by deficient mammary gland involution (Field et al., 2023; Lanctot et al., 2017). Immunity in the mammary glands is important for preventing bacterial infections. Immunity plays a crucial role during the dry period of the mammary glands. Therefore, the present study examined the effect of inflammatory status at the onset of cessation on changes in the concentration of antimicrobial components in milk during the subsequent 8 weeks.
During the dry period, healthy udders produce yellow and highly viscous milk (Watanabe et al., 2018). However, the precise changes in healthy and mastitic udders have not been examined. The present study investigated the color and viscosity of milk during the 8 weeks after cessation of milking and compared them between healthy and mastitic udders. At the onset of milking cessation, the color and viscosity scores of the milk did not differ between the groups. However, the color scores of milk at weeks 1, 3, and 4 were significantly lower in the LPS group than in the control group, and the viscosity scores at weeks 3 and 4 were significantly lower in the LPS group than in the control group. These results suggested that under high SCC conditions, color and viscosity do not change after the cessation of milking. In healthy udders, prolonged high pressure inside the udder weakens the integrity of tight junctions between epithelial cells, which induces water discharge from the inside to the outside of the alveolus, followed by an increase in the concentration of the milk components. (Pascottini et al., 2020; Zhao et al., 2019). This indicates that deficient milk production in mastitic udders cannot increase the intramammary pressure; therefore, the color and viscosity scores in the LPS group did not change during the experimental period.
In the present study, SCC increased after milking cessation and remained high in the control group, which is similar to the results of a previous study on mammary gland involution in cows (Zhao et al., 2019). However, the SCC decreased at week 1 and remained at low levels for 8 weeks in the LPS group. Milk in healthy udders is absorbed after milking cessation, resulting in a high SCC (Pascottini et al., 2020). However, in the LPS group, the lower intramammary pressure may fail to absorb milk, resulting in low SCC.
This study determined the concentration of certain antimicrobial components in milk during the 8 weeks after milking cessation. LF and S100A8 concentrations were higher in the control group than in the LPS group at 8 weeks, except for S100A8 at week 5. There were significantly higher concentrations of S100A8 at week 3 and LF at week 4 in the experimental group than in the control group. The Cath-7 level was also higher in the control group than in the LPS group at week 4. Cath-7 and S100A8 are secreted by leukocytes in response to intramammary stimulation with lipopolysaccharides (LPS) (Jahani et al., 2015; Nishikawa et al., 2018; Purba et al., 2019). These results suggested that the inflammatory state during milking cessation decreases the concentration of antimicrobial components, which may result in unsuccessful udder involution.
Cath-2, S100A8, and LF are synthesized by leukocytes and exhibit antimicrobial activity against a broad spectrum of bacteria (Isobe, 2017). Leukocytes are the major cell type involved in SCC (Dosogne et al., 2003; Sarikaya et al., 2006). Therefore, the increase in S100A8 and LF was due to high SCC after milking cessation. Therefore, a high SCC may be important for the defense system of the mammary glands during the dry period. However, Cath-2 cells exhibited the opposite effect. The concentration of Cath2 was higher in the LPS group than in the control group at weeks 0, 1, and 4. Cath-2 is secreted only by leukocytes and not by epithelial cells (Zhang et al., 2014). However, S100A8 and LF induce the production of leukocytes and epithelial cells (Conneely, 2001; Purba et al., 2019). These differences may have caused the opposite results in milk concentrations after cessation. It is possible that mammary epithelial cells contribute to the antimicrobial components concentration in milk until 4 weeks after the cessation of milking, even when epithelial cells are disappearing as described below. In this study, S100A7 concentration gradually decreased after milking cessation and showed no difference between the two groups. S100A7 is secreted mainly near teat skin and epithelial cells (Isobe, 2017). Therefore, S100A7 may play a weak role in preventing mammary infections during the dry period. This may be because involution occurs primarily in the udder parenchyma.
Four weeks after milking cessation, the lobule area in the LPS group was larger than that in the control group; conversely, the connective tissue area was smaller than that in the control group. Normal mammary involution is accompanied by the disappearance of lobules and the replacement of connective tissue (Masso-Welch et al., 2000). Therefore, inflammation during milking cessation prevents mammary gland involution, which may be due to the low intramammary pressure caused by the inflammatory condition, as described above (Fleet & Peaker, 1978).
In conclusion, the results of this study showed that the inflammatory state at milking cessation disrupts the mammary gland involution process and decreases some antimicrobial component concentrations compared to normal conditions. Therefore, animals with mastitis should recover prior to the onset of the dry period.
ACKNOWLEDGMENTS
This work was supported by JST, the establishment of university fellowships towards the creation of science technology innovation to J. S. (Grant Number JPMJFS2129).
CONFLICT OF INTEREST STATEMENT
All authors have no conflicts of interest.




