Condensed tannin-induced variations in the rumen metabolome and the correlation with fermentation characteristics in goats
Abstract
In this study, we characterized the effects of CT dietary inclusion at 2% (wt/wt) dry matter on the goat rumen metabolome and fermentation characteristics. Barley (BA) and corn (CN) were separately used as basal grain for the control rations, and rations supplemented with CT were BACT and CNCT, respectively. The rations were tested using eight Japanese Shiba × Saanen goats in a replicated 4 × 4 Latin square arrangement (28 days for each period). Ruminal fluid was obtained on day 25 of each period, and ultra-performance liquid chromatography–mass spectrometry (UPLC–MS) analysis was performed. Metabolites from BACT against BA and CNCT against CN were mostly associated with purine metabolism. Moreover, BACT against BA showed intensified biosynthesis of unsaturated fatty acids, and CNCT against CN resulted in strengthened amino acid metabolism. Furthermore, strong correlations were observed between rumen NH3-N and the copy number of total bacteria with most of the differential metabolites. The present paper provides a better understanding of the relationship between the rumen metabolome and fermentation characteristics and supports a shift in concern about using CT as a strategy to manipulate rumen metabolism.
1 INTRODUCTION
Adding plant polyphenols, such as condensed tannins (CT), in the ruminant diet has been widely tested over the past decades, and data have concentrated on nutritional outcomes (protein utilization, biohydrogenation, etc.; Barry & McNabb, 1999; Frutos et al., 2020) and health status (anti-bloat, antioxidation, etc.; Buccioni et al., 2017; Huang et al., 2018). Generally, CT exerts its functional effects by generating complex molecules (with nutrients or stimuli; Arts et al., 2002; Riedl & Hagerman, 2001) and interfering with specific microbial strains to induce remarkable alterations in their metabolites (Buccioni et al., 2015; Frutos et al., 2020). Moreover, because of high molecular weight and easy-to-form insoluble complexes with nutrient components, CT is basically nondegradable and poorly absorbable in the animal gastrointestinal tract (Koleckar et al., 2008). Among the CT origins, quebracho is the most studied cultivar thus far. Quebracho CT is widely applied to manufacture products and is utilized in livestock farming, especially in South and Latin America (Landau et al., 2000).
Earlier studies have already observed considerable effects of dietary CT on rumen fermentation. For instance, CT supplementation at 52.8 g/kg in the grazing ewe diet lowered the rumen propionate level (Buccioni et al., 2017), and an in vitro study showed that the growth of Streptococcus bovis was inhibited when CT was present in the culture (Min et al., 2003). Furthermore, Patra and Saxena (2011) indicated that dietary CT can also selectively decrease rumen enzyme activities. Considering that rumen fermentation supplies 70% to 80% of ruminant daily energy needs (Bergman, 1990), elucidating the variations in rumen metabolites may aid in understanding CT mechanisms on host nutrient metabolism. However, to our knowledge, a systematic overview regarding dietary CT inclusion in the rumen metabolome using soft/solid ionization (such as liquid chromatography/gas chromatography–mass spectrometry [LC/GC–MS]) and the correlation between the rumen metabolome and fermentation characteristics is limited.
Recently, Frutos et al. (2020) suggested that the impact of CT on host nutrient metabolism was also dependent on dietary components. In practical farming, ruminants consume a large amount of energy substrate to support production needs, and barley and corn, which differ in terms of starch and protein content, are the two major energy substrates widely used in ruminant feed (Nikkhah, 2012). The discrepancies in CT affinity with nutrients of these two cereal grains encouraged us to hypothesize that dietary CT inclusion would alter rumen fermentation characteristics, especially short-chain fatty acids, bacterial flora, and enzyme activity, and these alterations are strongly correlated with the rumen metabolome. Furthermore, we anticipated that these effects would vary depending on the dietary components, primarily because of differences in CT affinity with starch and protein in the gastrointestinal tract (Haddad & Nasr, 2007). This variation in affinity may result in differences between the metabolites generated in the rumen. We aimed to detect the variation of animal performance, rumen fermentation, and metabolome after CT supplementation, and clarify the networks between the rumen metabolome and fermentation characteristics under CT effects using goats as the animal model.
2 MATERIALS AND METHODS
2.1 Animal welfare statement
This study was approved by the Committee for Animal Research and Welfare of Gifu University (approval ID: #2021-175). All animal experimental procedures were conducted following the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, 2006; http://www.scj.go.jp/ja/info/kohyo/pdf/kohyo-20-k16-2e.pdf) and the Guidelines of Animal Research and Welfare of Gifu University (2008; https://www.gifu-u.ac.jp/20150821-12a-experi.pdf).
2.2 Animals, diets, and experimental design
In brief, a 2 × 2 factorial arrangement with two grain types and two CT levels was applied, resulting in four treatment diets: corn-based (CN) diet; corn-based with 2% (wt/wt) condensed tannin (CNCT) diet; barley-based (BA) diet; and barley-based with 2% (wt/wt) condensed tannin (BACT) diet. Experimental groups were stratified in a replicated 4 (diets) × 4 (squares, two goats per square) Latin square design using eight castrated, healthy male Japanese Shiba × Saanen goats of 2 years old with an average body weight of 34.4 ± 2.1 kg and similar body condition. Each square lasted 28 days, consisting of 21 days of adaptation and 7 days of sampling. The goats were kept in individual metabolic pens (0.8 × 1.2 m) throughout the study and fed at 09:00 and 19:00 daily with ad libitum access to water and mineral blocks. Experimental diets were formulated to have similar energy and protein levels (Table 1) that met the maintenance requirements according to the Nutritional Requirements of Small Ruminants (NRC, 2007). The zootechnical commercial quebracho condensed tannin (MGM-EX®, Unitan, Argentina) used was purchased as a powder that contained ≥75% CTs (other components, including catechin, epicatechin, gallocatechin, and epigallocatechin, were <15%).
| Diets | BA | CN |
|---|---|---|
| Ingredients (percent of dry matter) | ||
| Steam-flaked corn | - | 50.0 |
| Steam-flaked barley | 50.0 | - |
| Alfalfa hay | 10.2 | 10.2 |
| Sudangrass | 37.4 | 38.1 |
| Vegetable oil | 1.2 | - |
| Urea | - | 0.5 |
| Tricalcium phosphate | 0.5 | 0.5 |
| Salt | 0.5 | 0.5 |
| Vitamin premixa | 0.2 | 0.2 |
| Nutrients (percent of dry matter) | ||
| Metabolizable energy (MJ/kg)b | 11.0 | 11.0 |
| Crude protein | 11.3 | 11.3 |
| Ether extract | 4.2 | 4.1 |
| ADFom | 22.4 | 22.5 |
| aNDFom | 38.0 | 33.2 |
| Starch | 30.8 | 39.5 |
- Abbreviations: CN, corn-based diet; BA, barley-based diet; ADFom, ADF expressed exclusively of residual ash; aNDFom, neutral detergent fiber with heat-stable amylase expressed exclusively of residual ash.
- a Vitamin Premix consisted of the following per kg diet: vitamin A 1 × 107 IU, vitamin D3 2 × 106 IU, and vitamin E 1 × 104 IU.
- b Calculated according to Standard Tables of Feed Composition in Japan (NARO, 2009).
2.3 Sampling and analysis
Feed samples were collected every day during the sampling period, oven-dried at 60°C for 48 h, and ground through a 1-mm screen for analysis of dry matter (DM; 934.01), crude protein (CP; 984.13), crude ash (942.05), and ether extract (EE; 920.39; Association of Officiating Analytical Chemists [AOAC], 2005). The organic matter (OM) was calculated as the difference between DM and crude ash. The ADFom (acid detergent fiber expressed exclusive of residual ash) and aNDFom (neutral detergent fiber with heat-stable amylase and expressed exclusive of residual ash) were analyzed following Van Soest et al. (1991). Ruminal fluid samples were collected on day 25 of each square, 3 h after morning feeding, using an esophageal tube (Tian et al., 2019). After pH determination, ruminal fluid was collected into tubes specific for short-chain fatty acid (SCFA), microbial, and metabolome analysis.
The methane yield was calculated considering 90% hydrogen recovery according to Moss et al. (2000). Microbial DNA was extracted from a 0.5-mL homogenized sample (Yu & Morrison, 2004) and determined purity. Extracted DNA was quantified with specific primers for each targeted bacteria (Table S1) and qPCR SYBR Mix (Toyobo, Osaka, Japan), and established standard curve for each targeted microbe. Estimates of gene copy numbers for selected bacteria were log-transformed and presented as gene copy numbers per milliliter of fermentation fluid. The activity of bacterial enzymes, including α-amylase, cellulase, protease, and lipase, were measured following the method of Agarwal et al. (2002).
The pretreatment of ruminal fluid samples before SCFA and metabolome analyses followed the method described in our previous article (Aldian et al., 2023). The untargeted metabolome analyses were performed on an ultrahigh-performance liquid chromatography-tandem quadrupole time-of-flight mass spectrometer (UPLC-QTOF-MS, Xevo™ QToF; Waters, Milford, MA, USA) equipped with a C18 analytical column (ACQUITY UPLC BEH C18; 2.1 mm × 100 mm; Waters). The mobile phases consisted of 0.1% formic acid (A, diluted in UPLC grade ddH2O) and acetonitrile (B). The phase flow rate was 0.4 mL/min, and the sample injection volume was 5.0 μL. The elution gradient was 0 min, 95% A; 3 min, 80% A; 9–13 min, 5% A; and 13.10 min, 95% A, and held at 95% until 16 min. Electrospray ionization was operated in positive and negative modes. Raw data from the MS were converted into the .abf format using the AnalysisBaseFile Conventor (Reifycs Inc. Tokyo, Japan) and processed in MSDIAL (Ver. 4.9; Tsugawa et al., 2015). The retention time shift, mass accuracy, and relative standard deviation of peak areas were set at 0.3 min, 5 mDa, and 30%, respectively. The ions detected were matched with the in-house database of MSDIAL (Ver. Aug. 21st, 2022), and metabolites with a total score higher than 700 were considered identified metabolites. Subsequently, the results from MSDIAL were transformed into the .csv format, fed to MetaboAnalyst (Ver. 5.0; https://www.metaboanalyst.ca/; Pang et al., 2022), and then normalized using an internal standard and log transformation. Normalized data were employed in the SIMCA software package (Ver. 18; MKS Data Analytics Solutions, Umeå, Sweden) for supervised orthogonal partial least-squares discriminant analysis (OPLS-DA) and permutation tests of OPLS-DA results. To refine these results, a volcano plot in MetaboAnalyst was generated for a metabolite overview between the control and CT supplementation groups. Subsequently, the first principal component of variable importance in the projection (VIP), Student's t test, and log2-fold change (log2FC) were calculated. Metabolites with VIP values >1 and P values <0.05 were recognized as distinct between the control and CT supplementation groups. Finally, recognized metabolites were analyzed using Pathway Analysis embedded in MetaboAnalyst, and validation of the affected pathways was based on the Bovine Metabolome Database (BMDB) and Kyoto Encyclopedia of Genes and Genomes (KEGG).
2.4 Statistical analysis
3 RESULTS
3.1 Animal performance
Goats in the present experiment consumed all feed offered regardless of treatment because of restricted feeding and showed little variation in body weight after the experiment (Table 2; P > 0.05).
| Items | Corn-based diet | Barley-based diet | ||||||
|---|---|---|---|---|---|---|---|---|
| BA | BACT | SEM | P-value | CN | CNCT | SEM | P-value | |
| Animal performance | ||||||||
| DMI (kg) | 1.01 | 1.03 | 0.01 | 0.47 | 1.03 | 1.00 | 0.01 | 0.26 |
| Start weight (kg) | 34.91 | 34.58 | 0.78 | 0.84 | 34.65 | 34.29 | 0.35 | 0.62 |
| End weight (kg) | 33.98 | 35.56 | 0.81 | 0.35 | 35.13 | 34.06 | 0.33 | 0.10 |
| Weight change (kg) | 0.94 | −0.98 | 0.99 | 0.35 | 0.35 | −0.48 | 0.38 | 0.30 |
| Rumen fermentation | ||||||||
| pH | 6.39 | 6.21 | 0.05 | 0.04 | 6.09 | 6.10 | 0.08 | 0.95 |
| NH3-N (mg/dL) | 26.51 | 8.10 | 2.55 | < 0.01 | 42.43 | 26.83 | 2.28 | < 0.01 |
| Total SCFA (mmol/L) | 94.60 | 97.72 | 1.96 | 0.44 | 100.00 | 99.68 | 1.80 | 0.93 |
| Acetate (C2, mol/100 mol) | 65.00 | 66.81 | 0.78 | 0.26 | 66.39 | 66.40 | 0.67 | 0.99 |
| Propionate (C3) | 24.96 | 25.38 | 0.55 | 0.72 | 25.20 | 23.95 | 0.55 | 0.27 |
| Butyrate | 3.50 | 3.01 | 0.09 | < 0.01 | 3.84 | 4.26 | 0.16 | 0.20 |
| Valerate | 1.30 | 0.94 | 0.06 | < 0.01 | 1.32 | 1.37 | 0.03 | 0.46 |
| Iso-butyrate | 1.11 | 0.76 | 0.06 | < 0.01 | 1.15 | 1.24 | 0.07 | 0.50 |
| Iso-valerate | 3.90 | 3.14 | 0.17 | 0.02 | 2.00 | 2.34 | 0.09 | 0.06 |
| C2: C3 (mol/mol) | 2.63 | 2.66 | 0.09 | 0.85 | 2.67 | 2.78 | 0.08 | 0.46 |
| Methane (mmol/L) | 23.79 | 24.29 | 0.46 | 0.60 | 24.48 | 24.99 | 0.39 | 0.53 |
- Abbreviations: ADFom, ADF expressed exclusive of residual ash; aNDFom, NDF assayed with a heat stable amylase and expressed exclusive of residual ash; BA, barley-based diet; BACT, barley-based diet + 2% condensed tannin; CN, corn-based diet; CNCT, corn-based diet + 2% condensed tannin; CT, condensed tannin; Grain, grain source; SCFA, short-chain fatty acid; SEM, standard error of the mean.
3.2 Rumen fermentation characteristics
In the present study, the ruminal pH was lower in BACT than in BA (P = 0.04; Table 2) but not significant for CNCT against CN (P = 0.08). The content of NH3-N was lower after CT inclusion regardless of dietary basal grain (P < 0.01). The effect of CT supplementation on total SCFAs was not significant for the barley-based diet or corn-based diet (P > 0.05). However, individual SCFAs varied depending on the basal diet, with lower levels of butyrate, valerate, iso-butyrate, and iso-valerate in BACT compared to BA (P < 0.01), but little variation was noticed in CNCT compared to CN (P > 0.05). Acetate (C2) to propionate (C3) ratios were similar between the treatments (P > 0.05).
For bacterial flora (Table 3), CT inclusion decreased the abundance of total bacteria in both the barley-based diet and corn-based diet (P < 0.01). The abundance of S. bovis was lower in BACT than in BA (P < 0.01) but not significantly different in CNCT compared with CN (P > 0.05). Intriguingly, the copy number of Ruminococcus flavefaciens increased with CT in both the barley-based diet and corn-based diet (P < 0.05). Other tested bacteria, including Prevotella spp., Eubacterium ruminantium, Fibrobacter succinogenes, Selenomonas ruminantium, Prevotella ruminicola, Butyrivibrio fibrisolvens, and Ruminococcus albus, were similar between the treatments (P > 0.05). Enzyme activities, including α-amylase, protease, and carboxymethyl-cellulase, were decreased by CT in both the barley-based diet and corn-based diet (P < 0.05). However, the lipase activity was not significant for the barley-based diet or corn-based diet (P > 0.05).
| Items | Barley-based diet | Corn-based diet | ||||||
|---|---|---|---|---|---|---|---|---|
| BA | BACT | SEM | P-value | CN | CNCT | SEM | P-value | |
| Bacterial flora | ||||||||
Total bacteria (×1011 copies/mL) |
1.16 | 0.63 | 0.11 | < 0.01 | 1.24 | 0.70 | 0.09 | < 0.01 |
Prevotella spp. (×1010 copies/mL) |
2.17 | 2.12 | 0.17 | 0.90 | 2.01 | 1.76 | 0.24 | 0.62 |
| Amylolytic bacteria | ||||||||
E. ruminantium (×108 copies/mL) |
1.98 | 1.51 | 0.16 | 0.15 | 0.80 | 0.83 | 0.09 | 0.89 |
F. succinogenes (×107 copies/mL) |
4.27 | 3.39 | 0.64 | 0.51 | 4.91 | 4.23 | 0.56 | 0.57 |
S. bovis (×108 copies/mL) |
2.19 | 0.47 | 0.31 | < 0.01 | 2.90 | 2.13 | 0.32 | 0.24 |
S. ruminantium (×109 copies/mL) |
1.91 | 1.58 | 0.20 | 0.44 | 1.85 | 1.76 | 0.15 | 0.76 |
P. ruminicola (×109 copies/mL) |
4.48 | 3.93 | 0.52 | 0.62 | 5.40 | 4.23 | 0.62 | 0.36 |
| Cellulolytic bacteria | ||||||||
B. fibrisolvens (×109 copies/mL) |
8.33 | 7.11 | 0.11 | 0.60 | 6.31 | 6.26 | 0.77 | 0.98 |
R. albus (×106 copies/mL) |
5.61 | 5.70 | 0.07 | 0.95 | 6.49 | 5.74 | 0.63 | 0.57 |
R. flavefaciens (×107 copies/mL) |
1.00 | 1.41 | 0.09 | 0.01 | 0.76 | 1.29 | 0.13 | 0.03 |
| Enzyme activitya | ||||||||
| α-Amylase | 0.18 | 0.15 | 0.00 | < 0.01 | 0.25 | 0.23 | 0.01 | 0.02 |
| Protease | 0.80 | 0.60 | 0.04 | < 0.01 | 0.86 | 0.63 | 0.05 | < 0.01 |
| Caboxymethyl-cellulase | 1.51 | 1.38 | 0.03 | 0.04 | 1.74 | 1.61 | 0.03 | < 0.01 |
| Lipase | 0.17 | 0.17 | 0.00 | 0.08 | 0.13 | 0.13 | 0.00 | 0.76 |
- Abbreviations: BA, barley-based diet; BACT, barley-based diet + 2% condensed tannin; CN, corn-based diet; CNCT, corn-based diet + 2% condensed tannin; CT, condensed tannin; Grain, Grain source; SEM, stand error of the mean; F. succinogenes, Fibrobacter succinogenes; R. flavefaciens, Ruminococcus flavefaciens; E. ruminantium, Eubacterium ruminantium; P. ruminicola, Prevotella ruminicola; S. ruminantium, Selenomonas ruminantium; S. bovis, Streptococcus bovis; B. fibrisolvens, Butyrivibrio fibrisolvens; R. albus, Ruminococcus albus.
- a Units of enzyme activity are α-Amylose (μmol glucose min−1 mL−1); Protease (μmol L-tyrosine h−1 mL−1); Carboxymethyl-cellulase (μmol glucose h−1 mL−1); Lipase (μmol p-nitrophenol min−1 mL−1).
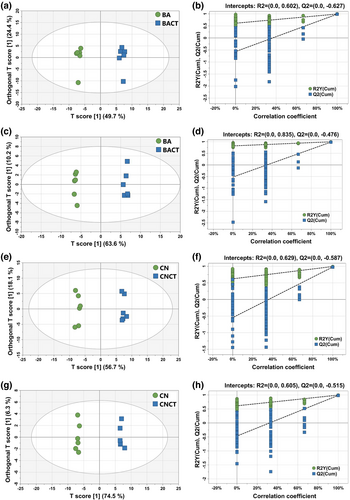
3.3 Multivariate and pathway variations
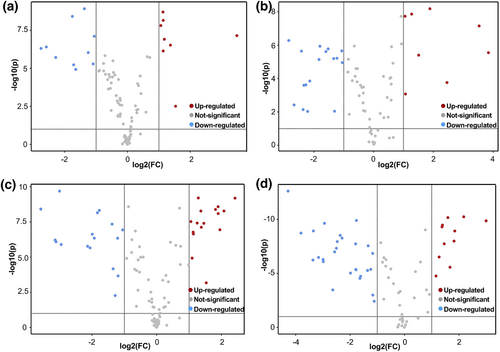
A total of 1642 and 571 MS peaks were identified in positive and negative ionizations for the ruminal fluid, respectively. The OPLS-DA score plots of all samples were inside the 95% Hotelling T-squared ellipse (Figure 1). As seen in Figure 1, there were distinct separations between the control and CT-supplemented groups in the OPLS-DA plots regardless of the MS mode. The R2Y intercepts of all permutation tests were higher than 0.3, and Q2 was close to −0.5, indicating that the OPLS-DA models had good interpretability and predictability. As visualized in the volcano plot for positive ionization, 18 metabolites were different in BACT compared with BA, and 33 metabolites were different in CNCT compared with CN (Figure 2a, c); for negative ionization, 25 metabolites were observed to be distinct in BACT compared with BA, and 31 distinct metabolites were observed in CNCT compared with CN (Figure 2b, d). After further filtration, the major differentially abundant metabolites resulting from CT inclusion in both the barley-based diet and corn-based diet (VIP > 1, P < 0.05) are shown in Table 4. Compared with BA, BACT had a higher concentration of linoleic acid (log2FC = 1.13) but a lower concentration of oleic acid (log2FC = 1.83). Higher levels of purine derivatives, including 2-deoxyadenosine (log2FC = 1.14), inosine (log2FC = 1.51), guanosine (log2FC = 1.88), and adenine (log2FC = 1.17), but lower levels of cyclic GMP (log2FC = −1.05) were observed. Goats fed the corn-based diet supplemented with CT showed significant alterations in purine derivatives, such as increased hypoxanthine (log2FC = 1.25) and inosine (log2FC = 1.67), with decreased guanine (log2FC = −1.34), cyclic AMP (log2FC = −3.58), cyclic GMP (log2FC = −3.35), guanosine (log2FC = −1.21), and adenine (log2FC = −2.29). Additionally, there were increases in amino acids such as L-phenylalanine (log2FC = 1.06), L-tyrosine (log2FC = 1.90), L-tryptophan (log2FC = 1.37), and phenylacetic acid (log2FC = 1.85), a carboxylic acid originating from amino acid metabolism, in CNCT. The metabolites with little difference after CT supplementation in the barley-based diet or corn-based diet are shown in Table S2.
| Metabolites | VIP | log2FC | P value | Metabolites | VIP | log2FC | P value |
|---|---|---|---|---|---|---|---|
| BACT/BA | Continued (CNCT/CN) | ||||||
| Positive | Hypoxanthine | 1.31 | 1.25 | < 0.001 | |||
| 6-pentyl-2H-pyran-2-one | 1.39 | 3.49 | < 0.001 | 4-methyl-5-thiazoleethanol | 1.30 | 1.13 | < 0.001 |
| Coniferaldehyde | 1.13 | 1.53 | 0.003 | 2-methylpyrrolidine | 1.30 | 1.13 | < 0.001 |
| 2,6-xylidine | 1.37 | 1.37 | < 0.001 | Quinoline | 1.24 | 1.09 | < 0.001 |
| Adenine | 1.38 | 1.17 | < 0.001 | L-phenylalanine | 1.31 | 1.06 | < 0.001 |
| 2-deoxyadenosine | 1.40 | 1.14 | < 0.001 | Isophorone | 1.30 | −1.05 | < 0.001 |
| Malonic acid | 1.37 | 1.14 | < 0.001 | 3′-adenylic acid | 1.16 | −1.19 | < 0.001 |
| Linoleic acid | 1.40 | 1.13 | < 0.001 | Guanosine | 1.28 | −1.21 | < 0.001 |
| Glycerol-myristate | 1.40 | 1.06 | < 0.001 | Coniferaldehyde | 1.00 | −1.28 | 0.005 |
| Proline | 1.39 | −1.06 | < 0.001 | 1-methylnicotinamide | 1.21 | −1.35 | < 0.001 |
| Raffinose | 1.35 | −1.09 | < 0.001 | Linoleic acid | 1.30 | −1.38 | < 0.001 |
| LPC 18:2 | 1.37 | −1.25 | < 0.001 | Trans-cinnamaldehyde | 1.31 | −1.77 | < 0.001 |
| Enterolactone | 1.40 | −1.37 | < 0.001 | Trehalose | 1.31 | −1.83 | < 0.001 |
| Tryptophol | 1.34 | −1.65 | < 0.001 | γ-Undecalactone | 1.29 | −1.93 | < 0.001 |
| Kynurenic acid | 1.36 | −1.72 | < 0.001 | Raffinose | 1.28 | −2.00 | < 0.001 |
| Trehalose | 1.41 | −1.76 | < 0.001 | Tryptophol | 1.27 | −2.06 | < 0.001 |
| Quinolone | 1.37 | −2.29 | < 0.001 | 6-pentyl-2H-pyran-2-one | 1.26 | −2.14 | < 0.001 |
| Oxindole-3-acetic acid | 1.38 | −2.59 | < 0.001 | Kynurenic acid | 1.28 | −2.95 | < 0.001 |
| Quinaldic acid | 1.38 | −2.76 | < 0.001 | 3-hydroxyanthranilic acid | 1.32 | −3.00 | < 0.001 |
| Negative | Quinaldic acid | 1.28 | −3.11 | < 0.001 | |||
| Glutaric acid | 1.19 | 3.84 | < 0.001 | (2-oxo-2,3-dihydro-1H-indol-3-yl) acetic acid | 1.28 | −3.14 | < 0.001 |
| Chrysanthemic acid | 1.22 | 3.53 | < 0.001 | Adenosine 2′,3′-cyclic phosphate | 1.31 | −3.58 | < 0.001 |
| Dodecanedioic acid | 1.10 | 2.46 | < 0.001 | Cyclic AMP | 1.31 | −3.58 | < 0.001 |
| Guanosine | 1.24 | 1.88 | < 0.001 | Negative | |||
| Oleic acid | 1.19 | 1.83 | < 0.001 | Hypotaurine | 1.15 | 3.00 | < 0.001 |
| Inosine | 1.20 | 1.51 | < 0.001 | Phenylacetic acid | 1.15 | 1.85 | < 0.001 |
| Ethyldodecanoate | 1.24 | 1.27 | < 0.001 | Inosine | 1.10 | 1.67 | < 0.001 |
| Sotalol | 1.03 | 1.06 | < 0.001 | Oxypurinol | 1.15 | 1.59 | < 0.001 |
| Undecanedioic acid | 1.23 | 1.05 | < 0.001 | Medicagenic acid | 1.15 | 1.39 | < 0.001 |
| Cyclic GMP | 1.18 | −1.05 | < 0.001 | Andrastin A | 1.12 | 1.27 | < 0.001 |
| 2′-deoxyguanosine 5′-monophosphate | 1.21 | −1.07 | < 0.001 | 2,8-quinolinediol | 1.06 | 1.16 | < 0.001 |
| LPC 16:0 | 1.19 | −1.23 | < 0.001 | 2′-deoxyguanosine 5′-monophosphate | 1.00 | −1.12 | 0.004 |
| 2,8-quinolinediol | 1.00 | −1.31 | < 0.001 | Uridine 5′-monophosphate | 1.00 | −1.17 | 0.001 |
| LPE 18:2 | 1.18 | −1.34 | < 0.001 | Decanedioic acid | 1.14 | −1.17 | < 0.001 |
| Pyrocatechol | 1.20 | −1.52 | < 0.001 | LPE 18:2 | 1.10 | −1.24 | < 0.001 |
| 1H-benzotriazole-5-carboxylic acid | 1.22 | −1.80 | < 0.001 | Guanine | 1.14 | −1.34 | < 0.001 |
| Oxymetholone | 1.15 | −2.14 | < 0.001 | Pentadecanoic acid | 1.09 | −1.36 | < 0.001 |
| D-glucuronolactone | 1.00 | −2.21 | 0.009 | D-glucuronolactone | 1.08 | −1.61 | < 0.001 |
| Trifluoroacetic acid | 1.12 | −2.27 | < 0.001 | Benzenesulfonic acid | 1.07 | −1.64 | < 0.001 |
| Propiolate | 1.11 | −2.33 | < 0.001 | Cholate | 1.15 | −1.77 | < 0.001 |
| Benzenesulfonic acid | 1.00 | −2.36 | < 0.001 | Trifluoroacetic acid | 1.10 | −1.78 | < 0.001 |
| 3-methyl-2-oxindole | 1.22 | −2.41 | < 0.001 | Propiolate | 1.10 | −1.79 | < 0.001 |
| Ethylmalonic acid | 1.00 | −2.66 | 0.004 | Pyrocatechol | 1.14 | −1.80 | < 0.001 |
| 4-hydroxyquinoline | 1.23 | −2.84 | < 0.001 | Ethylmalonic acid | 1.10 | −1.99 | < 0.001 |
| CNCT/CN | Adenine | 1.14 | −2.29 | < 0.001 | |||
| Positive | Undecanedioic acid | 1.14 | −2.47 | < 0.001 | |||
| 2,6-xylidine | 1.32 | 2.42 | < 0.001 | 13-HODE | 1.14 | −2.50 | < 0.001 |
| Benzimidazole | 1.32 | 2.08 | < 0.001 | Dodecanedioic acid | 1.13 | −2.57 | < 0.001 |
| Thymine | 1.30 | 1.95 | < 0.001 | Chrysanthemic acid | 1.00 | −2.64 | < 0.001 |
| Azelaic acid | 1.30 | 1.90 | < 0.001 | Oxymetholone | 1.15 | −2.94 | < 0.001 |
| L-tyrosine | 1.32 | 1.90 | < 0.001 | Methyl oxindole-3-acetate | 1.11 | −2.99 | < 0.001 |
| Malonic acid | 1.31 | 1.82 | < 0.001 | Methylsuccinic acid | 1.11 | −2.99 | < 0.001 |
| Resveratrol | 1.11 | 1.53 | < 0.001 | Cyclic GMP | 1.15 | −3.35 | < 0.001 |
| DL-norleucine | 1.31 | 1.38 | < 0.001 | 4-hydroxyquinoline | 1.11 | −3.37 | < 0.001 |
| L-tryptophan | 1.32 | 1.37 | < 0.001 | Sotalol | 1.15 | −4.28 | < 0.001 |
| Tricin | 1.32 | 1.29 | < 0.001 | ||||
- Abbreviations: AMP, adenosine monophosphate; BA, barley-based diet; BACT, barley-based diet + 2% condensed tannin; CN, corn-based diet; CNCT, corn-based diet + 2% condensed tannin; GMP, guanosine monophosphate; HODE, hydroxyoctadecadienoic acid; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; VIP, variable importance in projection.
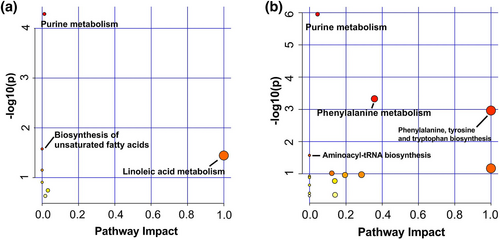
After mapping all the different metabolites to the KEGG database, we observed that the barley-based diet supplemented with CT remarkably impacted the biosynthesis of unsaturated fatty acids and linoleic acid metabolism (Figure 3a). The corn-based diet with CT significantly impacted not only phenylalanine metabolism and phenylalanine, tyrosine, and tryptophan biosynthesis but also aminoacyl-tRNA biosynthesis (Figure 3b). Both the barley-based diet and corn-based diet supplemented with CT affected purine metabolism.
3.4 Correlations between metabolome and fermentation characteristics
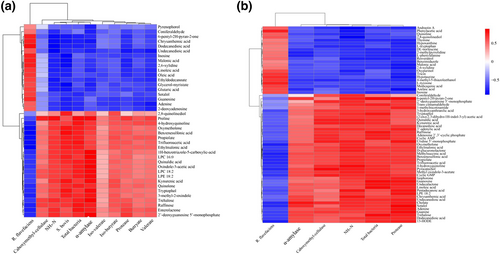
As shown in Figure 4a, in goats fed barley-based diets, guanine and adenine were negatively correlated with NH3-N, total bacteria, S. bovis, and α-amylase (r = −0.78, P < 0.05), but only guanine was positively correlated with R. flavefaciens (r = 0.57, P < 0.05). Linoleic and oleic acids were positively correlated with R. flavefaciens (r = 0.70, P < 0.05) but negatively correlated with NH3-N, total bacteria, S. bovis, and α-amylase (r = −0.71, P < 0.05). Goats fed corn-based diets displayed more significant correlations between metabolites and fermentation indices on the heatmap (Figure 4b), in which guanine and adenine were positively correlated with NH3-N, total bacteria, and protease (r = 0.65, P < 0.05) but negatively correlated with R. flavefaciens (r = −0.65, P < 0.05). Phenylalanine, tryptophan, and tyrosine were negatively correlated with NH3-N, total bacteria and protease (r = −0.60, P < 0.05), and only tyrosine was positively correlated with R. flavefaciens (r = 0.76, P < 0.01).
4 DISCUSSION
The progress made thus far suggests that condensed tannins (CT) can not only interfere with dietary protein but also selectively suppress the proliferation of specific rumen bacterial strains to manipulate nutrient metabolism (Frutos et al., 2020). In the present study, the fermentation characteristics of the two grain-based diets exhibited differences, despite both showing a degree of suppression. Unexpectedly, the inclusion of CT in the barley-based diet led to a considerable reduction in rumen SCFAs, whereas little effect was observed in the case of the corn-based diet. According to Nikkhah (2012), barley contains more amylopectin, which enables a higher ruminal digestion rate than corn. Hence, the decrease in SCFAs in BACT compared with BA might be attributed to the high digestion rate of barley, facilitating the effective binding of CT with released nutrients, thereby rendering them less available for microbial utilization. Min et al. (2003) reported that dietary CT can decrease microbial activity. Indeed, the abundance of total bacteria was reduced in both diets, and the decreased NH3-N, α-amylase, protease, and carboxymethyl-cellulase in both the barley-based diet and corn-based diet after inclusion with CT also indicate limited microbial activity (Yáñez-Ruiz et al., 2004). Intriguingly, the abundance of R. flavefaciens increased in both diets after CT supplementation. According to Costa et al. (2021), R. flavefaciens is a proteolytic microbe that is gram-positive and usually not tolerant to CT. However, Min et al. (2012) observed an increase in R. flavefaciens in the rumen under CT inclusion as the experimental time progressed. Thus, the possible explanation for this increase in R. flavefaciens abundance lies in the enhanced tolerance developed over the longer experimental period (4 weeks) in our study compared to others (2 to 3 weeks). It should be noted that although the statistical difference between other microbes were not significant (P > 0.05), the variance (or biological difference; e.g., the 24.7% of difference in E. ruminantium between BA (1.98 × 108 copies/mL) and BACT (1.51 × 108 copies/mL) cannot be ignored. However, the limit animal (eight goats in total) restricted the further explanation on these results; more study is still needed in clarifying the CT's impact on specific microbes. While it may appear that CT indiscriminately binds with nutrients to hinder its utilization, the unaffected body weight throughout the trial convinced us that, at least, dietary 2% CT was not unfavorable to goat's growth performance. This is in line with the conclusion that dietary inclusion of CT at an appropriate ratio (2–3% of dietary dry matter) is safe for animal health (Henke et al., 2017; Patra & Saxena, 2011). Of note, previous researches indicated that CT inclusion in the ruminant diet is capable of mitigate gastrointestinal methane production (Beauchemin et al., 2022; Buccioni et al., 2015). Unfortunately, this was not the case in our experiment. One of the vital mechanism of CT to reduce methane production was the reduction on acetate and butyrate production (Beauchemin et al., 2022). However, we did not observe significant change in ruminal acetate after inclusion of CT, neither in BA nor in CN. Although the butyrate was reduced in BACT compared with BA, the extent change may too small to arouse significant difference in methane production. Also, a high grain ratio in the diet are expected to produce a large volume of propionate, and production process of propionate will consume hydrogen used for methanogenesis (Jia et al., 2023). Therefore, the 50% of grain in both BA and CN diets in our study probably suppressed rumen methane production from the first place.
According to the KEGG pathway analysis, both the barley-based diet and corn-based diet supplemented with CT significantly impacted purine metabolism. Purine metabolism involves the conversion of adenine and guanine into uric acid through a series of metabolic enzymes (Tie et al., 2023). In ruminants, these metabolites are predominantly generated during microbial protein synthesis (Chen & Gomes, 1992), and the correlation analysis also displayed a strong negative and positive correlation in the barley-based diet and corn-based diet, respectively, between NH3-N and adenine and guanine. Therefore, the reduced levels of adenine and guanine in CNCT against CN indicate decreased microbial protein synthesis, which corresponded with the suppressed microbial activity. However, the higher levels of adenine and guanine in BACT compared with BA were contradictory to the detrimental effect of CT on microbial activity. The in vitro study of Castro-Montoya et al. (2011) also observed increases in adenine and guanine when 1 mg/mL quebracho CT was applied to the culture. Combined with the conclusion that purine derivative content differs with microbial types (Rodriguez et al., 2000), Castro-Montoya et al. (2011) attributed the increase in adenine and guanine to CT's selective inhibition of some microbial strains (same as that concluded by Buccioni et al., 2015; Frutos et al., 2020). Indeed, the detected bacterial strains also showed discrepancies in response to CT inclusion. Therefore, CT in the barley-based diet possibly elevated adenine and guanine levels by different microbial abundances compared with those in the corn-based diet.
The increase in linoleic acid and oleic acid and the intensified biosynthesis of unsaturated fatty acids and the metabolism of linoleic acid pathways by CT in the barley-based diet seem to have two explanations. The first and most appropriate one was the inhibitory effect of CT on ruminal biohydrogenation. Unsaturated fatty acids (such as 18:1 and 18:2) are beneficial for human health but strongly toxic to ruminal microbes (Keweloh & Heipieper, 1996). By selectively suppressing the proliferation of some bacterial strains, CT can interrupt the bacterial breakdown of the “double bond” on unsaturated fatty acids to weaken the process of biohydrogenation, therefore permitting the ruminal retention of these fatty acids (Frutos et al., 2020). It is already a common practice to use CT to enhance unsaturated fatty acids in ruminant products (Kronberg et al., 2007). In contrast, retention of these fatty acids will also inhibit the utilization of lysophosphatidylcholine (LPC) and lysophosphatidylethanolamine (LPE) in a negative feedback manner (Wang et al., 2022). Moreover, the inhibitory effect of CT on microbes might also be the reason that α-amylase (the primary enzyme responsible for the production of glucose, which is a crucial energy source for microbes) exhibited a negative correlation with both linoleic acid and oleic acid. The second explanation was that the intensification of body synthesis of unsaturated fatty acids compensated for rumen mobilization of body fats to counteract the inhibitory effect of CT on feasible energy substrates because of the suggestion that unsaturated fatty acids constitute more than half of animal fat and are particularly efficient in providing energy (Wood et al., 2004). Liu et al. (2018, 2023) measured the plasma metabolome in pigs under a maintenance state compared to those in an ad libitum-fed state and observed upregulated linoleic acid and oleic acid levels with an impacted linoleic acid metabolism pathway. This may also explain the reduction in the LPC levels 16:0, LPC 18:2, and LPE 18:2, which are metabolites produced from phosphatidylcholine hydrolysis (Schmitz & Ruebsaamen, 2010).
In the present study, the relative concentrations of phenylalanine, tryptophan, and tyrosine were upregulated in CNCT compared with CN. Tyrosine is a ketogenic amino acid that can be oxidized to meet body energy requirements (Balasse & Féry, 1989; Tanaka & Ichihara, 1975). However, there is no indication that the energy distribution was compromised following CT inclusion in the diet of corn-fed goats. Instead, phenylalanine, tryptophan, and tyrosine are associated with lipid synthesis (Flydal & Martinez, 2013). Therefore, the simultaneous increase in these amino acids is suggested as CT's manipulation of rumen lipid metabolism, also aligning with the conclusion drawn regarding CT's impact on the rumen biohydrogenation process (Frutos et al., 2020). To our knowledge, the present study was the first to demonstrate the variations in the rumen metabolome and fermentation characteristics under CT intervention in different grain-based diets. Indeed, the response from the two diets was distinct. Soldado et al. (2021) suggested that the imparity in host response after CT intervention in diets could be ascribed to multiple linkages involved in the integrated and synergistic cooperation among CT, diet, and host. One valid factor, as mentioned above, was the disparity in digestive rate between corn and barley, which can induce different timings for CT to exert its function. Moreover, the different CT affinities with dietary components (high affinity with fiber but low affinity with starch; Seifried et al., 2017) should also be considered. Finally, rapid rumen digestion makes a barley-based diet seem much more vulnerable to nutrient utilization than a corn-based diet because of the wide range of decreases in SCFAs. Thus, the enhancement of purine metabolism and linoleic acid metabolism after CT supplementation in barley-based diet-fed goats can be seen more as a “remediation” rather than a “manipulation” in the corn-based diet.
In conclusion, dietary CT inclusion considerably affected goat rumen fermentation characteristics and metabolome, and the effect was dependent on dietary basal grain. In summary, CT inclusion showed a remarkable impact on microbial protein synthesis by intensifying purine metabolism. CT supplementation in a barley-based diet resulted in enhanced unsaturated fatty acid levels as compensation for decreased α-amylase activity. The inclusion of CT in a corn-based diet displayed potential manipulation of lipid metabolism by upregulating amino acid synthesis. Therefore, the results from the present study may contribute to a better understanding of the relationship between rumen fermentation and the metabolome and support a shift in concern about using CT as a strategy to manipulate rumen metabolism.
ACKNOWLEDGMENTS
This work was funded by The United Graduate School of Agricultural Science of Gifu University and partially supported by a scholarship from Chinese Scholarship Council (CSC, Grant No. 202006990023) awarded to K.E. Tian. The authors would like to thank Professor Takashima Shigeo at Life Science Research Centre, Gifu University, for his kind assistance during the qPCR and LC–MS analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests for this article.




