Low dietary carbohydrate induces structural alterations in enterocytes of the chicken ileum
Abstract
We investigated the role of dietary carbohydrates in the maintenance of the enterocyte microvillar structure in the chicken ileum. Male chickens were divided into the control and three experimental groups, and the experimental groups were fed diets containing 50%, 25%, and 0% carbohydrates of the control diet. The structural alterations in enterocytes were examined using transmission electron microscopy and immunofluorescent techniques for β-actin and villin. Glucagon-like peptide (GLP)-2 and proglucagon mRNA were detected by immunohistochemistry and in situ hybridization, respectively. Fragmentation and wide gap spaces were frequently observed in the microvilli of the 25% and 0% groups. The length, width, and density of microvilli were also decreased in the experimental groups. The experimental groups had shorter terminal web extensions, and there were substantial changes in the mitochondrial density between the control and experimental groups. Intensities of β-actin and villin immunofluorescence observed on the apical surface of enterocytes were lower in the 0% group. The frequency of GLP-2-immunoreactive and proglucagon mRNA-expressing cells decreased with declining dietary carbohydrate levels. This study revealed that dietary carbohydrates contribute to the structural maintenance of enterocyte microvilli in the chicken ileum. The data from immunohistochemistry and in situ hybridization assays suggest the participation of GLP-2 in this maintenance system.
1 INTRODUCTION
Digestion and absorption of ingested nutrients occur in the small intestine, which is lined with absorptive epithelial cells, called enterocytes. These cells are involved in transporting nutrients from the lumen of the intestine to the circulatory system. Numerous tiny fingerlike projections, called microvilli, uniformly cover the apical domain of the enterocytes. Microvilli increase the free surface area of enterocytes, thereby improving the absorptive efficiency of the luminal nutrients (Crawley et al., 2014). Morphological features of the intestinal epithelium reflect the luminal state of the digestive organs because microvilli are in direct contact with the luminal content.
Various cytostructure proteins influence the maintenance of the shape and stability of the microvilli for optimal functionality. Among these proteins, actin provides support and forms the core bundle, consisting of approximately 20–30 actin filaments with some actin-bundling proteins (Ohta et al., 2012). Villin is a main actin-bundling protein in the microvilli (Coluccio & Bretscher, 1989) and terminal web (Khurana & George, 2008). The terminal web is a cytoplasmic area just beneath the microvilli and rich in actin filaments, which originate from both the microvilli core and adherens junctions. Microvilli are anchored to the terminal web, which functions as a contractile structure and affects the microvilli distribution pattern. Several studies have revealed that the structural characteristics of microvilli are not static (Stidwill et al., 1984) and vary according to the state of the actin and villin proteins. Several in vitro and in vivo studies have found that alterations in the actin microfilaments and levels of villin modify the cytoskeleton of the microvilli core and alter their structural features (Friederich et al., 1999; Revenu et al., 2012). It was demonstrated that adding and removing actin microfilaments from the cytoskeleton manipulated the shape of the microvilli (Mooseker & Tilney, 1975). However, another study indicated that the turnover rate of the microvilli cytoskeletal core components was rapid in vivo (Stidwill et al., 1984). Therefore, the state of actin and villin proteins regulates the steady condition of microvilli. The homeostasis of cytoplasmic organelles and microvilli components in enterocytes is an energy-consuming process. Mitochondria, commonly known as the powerhouse of the cell, generate energy for all cellular activities. In response to external stimuli, it is a critical site for signaling pathways where the signal molecules are generated. However, the number of mitochondria and their interactions with other cellular organelles determine cellular functional diversity. The biosynthesis and activities of mitochondria are essential for epithelial cell homeostasis in the gastrointestinal tract (Rath et al., 2018).
Glucagon-like peptide (GLP)-2 is a 33-amino-acid peptide that originates from proglucagon (PG) and is secreted from the intestinal L cells in response to ingested nutrients (Drucker et al., 1996; Xiao et al., 1999). The L cells in the chicken intestinal epithelium are open-type endocrine cells with a long cytoplasmic process that reaches the intestinal lumen (Nishimura et al., 2013). These cells act as sensors that monitor the ingested nutrients in the intestinal lumen. Some studies have shown that GLP-2 has many physiological functions in enterocytes, such as promoting proliferation and suppressing apoptosis (Burrin et al., 2003; Drucker et al., 1996). Thus, the maintenance of epithelial integrity for intestinal growth and the absorption of nutrients is an important function of GLP-2. In the last few decades, much interest has been directed in the structural arrangement of cytoskeletal core proteins that control the shape and stability of the microvilli. However, the role of dietary nutrients in the maintenance of intestinal epithelia, especially enterocyte microvilli, in both mammalian and avian species remains unknown. Dietary carbohydrates (CHOs) are the main energy source for domestic animals and poultry. Our recent study demonstrated that low dietary CHOs induced alterations in the morphology of the villi in the chicken small intestine (Salahuddin et al., 2021). This suggests that dietary CHOs are essential for the maintenance of small intestinal epithelial morphology and play an important role in the absorptive function. Therefore, the present study aimed to elucidate the importance of dietary CHOs in maintaining the structural integrity of enterocyte microvilli in the chicken ileum using transmission electron microscopy and immunohistochemistry. The involvement of GLP-2 in the maintenance of intestinal epithelium was investigated using immunohistochemistry for the GLP-2 peptide, in situ hybridization for PG mRNA, and morphometrical techniques.
2 MATERIALS AND METHODS
The chicken care and experimental protocols of this study were reviewed by the Committee for Animal Experiments of Shinshu University, and approval was granted by the president of Shinshu University (approval number 021013).
2.1 Animals and feeding management
Healthy male White Leghorn chickens (n = 20, 6-week-old, average weight of 581.1 ± 17.5 g) were used in this study. They were randomly separated into the control and three experimental groups (50%, 25%, and 0% groups), with five individuals in each group according to their average body weight. A control diet was provided to all chickens for 3 days in order to habituate the chickens to the diet. The experimental feeding started with each experimental diet shown in Table 1 under 12/12-h light/darkness cycle conditions for 7 days. The chickens were reared in individual cages (24 cm wide × 39 cm deep × 45 cm high) with ad libitum access to feed and water. The daily feed intake and body weight of each chicken were measured at the same clock time throughout the experimental period. The CHO content of the experimental diets for the 50%, 25%, and 0% groups was 50%, 25%, and 0% of that of the control diet, respectively. The control and experimental diets had the same CHO source (cornstarch). Furthermore, the metabolizable energy (ME) in each group was adjusted to a similar level by adding corn oil. Therefore, the ME level (2850 kcal/kg) of the diet was similar to that of the ME requirement, as determined by the Japanese Feeding Standard for Poultry.
| Composition | Treatments | |||
|---|---|---|---|---|
| Control | 50% | 25% | 0% | |
| Isolated soybean protein | 217.4 | 217.4 | 217.4 | 217.4 |
| l-Cysteine | 0.7 | 0.7 | 0.7 | 0.7 |
| l-Methionine | 0.9 | 0.9 | 0.9 | 0.9 |
| l-Threonine | 0.4 | 0.4 | 0.4 | 0.4 |
| Cornstarch | 491.4 | 245.7 | 122.9 | 0.0 |
| Cellulose | 194.7 | 340.2 | 412.9 | 485.7 |
| Corn oil | 30.0 | 130.2 | 412.9 | 485.7 |
| Mineral mixture | 60.0 | 60.0 | 60.0 | 60.0 |
| Vitamin mixture | 2.0 | 2.0 | 2.0 | 2.0 |
| Choline chloride | 1.5 | 1.5 | 1.5 | 1.5 |
| Inositol | 1.0 | 1.0 | 1.0 | 1.0 |
| Total (g) | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| ME (kcal/kg) | 2850 | 2850 | 2850 | 2850 |
| CP (%) | 18.0 | 18.0 | 18.0 | 18.0 |
- Note: Isolated soybean protein contains 82.8% of crude protein (CP).
- Abbreviation: ME, metabolizable energy.
2.2 Tissue samples
The chickens were intravenously injected with sodium pentobarbital (64.8 mg/kg body weight) and sacrificed by decapitation. The distal part of the ileum, in which the L cells are the most densely distributed in the chicken small intestine (Hiramatsu et al., 2005), was immediately dissected out of each chicken as a tissue sample, and its luminal contents were washed out with a 0.75% sodium chloride solution. For light microscopy, the tissue samples were fixed in Bouin's solution for 24 h and embedded in paraffin wax in an ordinary manner. For electron microscopy, a part of the tissue sample from three chickens from each group was randomly selected and cut into small pieces of less than 1 mm3 using a razor blade. This was transferred into 1/2 Karnovsky's fixative solution (a mixture of 2% paraformaldehyde and 2% glutaraldehyde in 50 mM of cacodylate buffer) at 4°C for 2 h. After washing with 50 mM of cacodylate buffer, the tissue samples were postfixed with 1% osmium tetroxide in 0.2 M of phosphate buffer at 4°C for 2 h and embedded in epoxy resin (Quetol 812; Nisshin EM, Tokyo, Japan).
2.3 Electron microscopy
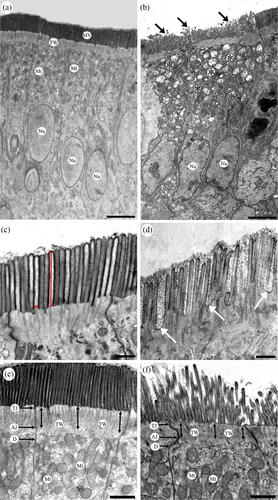
Ultra-thin sections were cut from each epoxy block using an ultramicrotome (SuperNova, Reichert-Jung, Vienna, Austria) and treated with 10% (v/v) TI Blue (Nisshin EM) and 2% (w/v) lead citrate for electron staining. These sections were observed and photographed under a transmission electron microscope (TEM; JEM-1400; JEOL, Tokyo, Japan). The length, width, and linear density of the enterocyte microvilli, which are known as the epithelial enlargement factors, were measured from the TEM images that were taken at 4000× magnification using ImageJ software (Fiji, National Institutes of Health, Bethesda, MD, USA). The length of a microvillus was measured from the tip to the bottom of each microvillus on the TEM images (Figure 1c, vertical double-headed red arrows), while the width of a microvillus was measured at the bottom position of each microvillus in the same set of images (Figure 1c, horizontal double-headed arrows). Moreover, 300 microvilli were randomly selected from multiple enterocytes of each chicken, and 900 microvilli were measured from the three chickens in each group for the measurement of the length and width. The linear density, which is the number of microvilli per micrometer in length of the apical surface of the enterocytes, was estimated by randomly choosing 40 areas of the apical surface of uninterrupted enterocytes from each chicken. A total of 120 areas were measured for the three chickens in each group. The number of microvilli per micrometer for each group was then calculated. The thickness of the terminal web was measured based on a previously reported method (Beer et al., 2020) with minor modifications. The distance from the plasma membrane to the adherens junction at the base of the microvilli was measured at three different points: both sides and the middle part of the apical surface of each enterocyte (Figure 1e,f, vertical double-headed arrows), to determine the terminal web thickness using ImageJ software. Fifty enterocytes from three chickens in each group were randomly chosen on the TEM images, and the final assessment of the length of this parameter was performed for 150 enterocytes from each group. The mitochondrial density in the enterocytes based on TEM images was measured using a previously reported method (Shini et al., 2021). The area of the apical cytoplasm (above the nucleus) of the enterocytes was calculated, and the number of mitochondria in this region was counted. The number of mitochondria per 100 μm2 of the apical area was calculated as the mitochondrial density.
2.4 Immunofluorescence for villin and β-actin
Immunofluorescence was performed to detect the immunoreactivity of β-actin and villin in the distal ileum. Paraffin sections were treated with 10% normal goat serum and incubated with mouse monoclonal antibody against villin (sc-58897, diluted to 1:20; Santa Cruz Biotechnology, Dallas, TX, USA) or rabbit antiserum against β-actin (GTX109639, 1:5000; Gene Tex, Irvine, CA, USA) for 24 h. After several washes with phosphate-buffered saline (PBS), the sections were incubated with DyLight 488-labeled goat anti-mouse IgG antibody (610-141-121, 1:300; Rockland, PA, USA) or DyLight 549-labeled goat anti-rabbit IgG antibody (611-142-122, 1:300; Rockland) for 3 h. Subsequently, these sections were covered with an aqueous mounting medium (Perma Fluor; Thermo Fisher Scientific, Fremont, CA, USA), shielded with a glass coverslip, and observed and photographed under a fluorescence microscope (AxioImagerA1; Carl Zeiss, Göttingen, Germany). All incubations were conducted in a moisture chamber at room temperature. The specificities of the primary antibodies used in this study were determined by the manufacturers.
2.5 Immunohistochemistry for GLP-2
The streptavidin-biotin method was used to identify the GLP-2-immunoreactive cells in the distal ileum. After treatment with an antigen retrieval agent (ImmunoSaver, Nisshin EM) at 98°C for 45 min, the paraffin sections were incubated with 10% normal goat serum (50062Z; Invitrogen, Carlsbad, CA, USA) for 20 min, followed by the incubation with rabbit anti-human (Arg34)-GLP-2 serum (1:2000, H-028-14; Phenix Pharmaceuticals, Burlingame, CA, USA) as a primary antibody for 24 h. After several washes with PBS, the sections were incubated with biotin-labeled goat anti-rabbit IgG serum (1:300, AP132B; Millipore, CA, USA), followed by poly-HRP20-labeled streptavidin (1:300, SP20C; Stereospecific Detection Technologies, Baesweiler, Germany). The immunocomplexes were visualized with a 0.05% 3,3′-diaminobenzidine solution in tris–HCl buffer. The sections were covered with a glass coverslip after counterstaining with Mayer's hematoxylin, and they were observed and photographed under a light microscope. The specificity of the primary antibody used in this study was tested in our previous study (Nishimura et al., 2013). All incubations were conducted in a moisture chamber at room temperature.
The morphometrical evaluation of the GLP-2-immunoreactive cells was performed using a computerized image analysis system (KS400; Carl Zeiss) according to our previously reported method (Hiramatsu et al., 2005). To assess the frequency of occurrence, the number of GLP-2-immunoreactive cells with clearly visible nuclei was counted. Subsequently, the mucosal layer area was measured to calculate the number of cells per mucosal area (cells/mm2). Twenty areas were randomly selected from each chicken, and 100 areas were measured from five chickens in each group for morphometrical analysis.
2.6 In situ hybridization for PG mRNA
In situ hybridization was performed to identify cells that expressed PG mRNA signals according to a previously reported method (Nishimura et al., 2016). A commercially available kit (IsHyb In Situ Hybridization Kit; Biochain Institute, Newark, CA, USA) was used to hybridize and visualize digoxigenin (DIG)-labeled probes in accordance with the manufacturer's instructions. Sections were incubated with an anti-DIG antibody diluted in an alkaline–phosphatase solution (1:500) for 1.5 h to overnight. The conjugated probe was visualized with a mixture of nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate. All oligonucleotide probes were designed according to the PG mRNA sequence (Nishimura et al., 2016). These probes were commercially synthesized (BEX, Tokyo, Japan) for this study. The sequences for these PG mRNA antisense and sense probes were 5′-GCTGTAGTCACTGGTGAATGTGCCTTGTGAATGACGCTTTA-3′ and 5′-TAAAGCGTCATTCACAAGGCACATTCACCAGTGACTACAGC-3′, respectively. Notably, a sense probe was applied as a negative control for the PG mRNA. The cells that expressed PG mRNA signals were counted using a computerized image analysis system, and the frequency of these cells per mucosal area (cells/mm2) was determined. Twenty areas were randomly selected from the preparations of each chicken, and 100 areas from five chickens of each group were examined to calculate the frequency of cells that expressed PG mRNA signals.
2.7 Statistical analysis
All statistical analysis was performed using SAS software (SAS Inst. Inc., Cary, NC, USA). The generalized linear model was performed using one-way analysis of variance (ANOVA). When statistically significant effects were detected by ANOVA, Tukey's honestly significant difference pair-wise comparisons were performed as post hoc analysis to evaluate the main effects of the dietary CHOs in the datasets of feed intake, body weight gain, electron microscopy parameters, and frequency of GLP-2-immunoreactive cells and cells that expressed PG mRNA signal. All variables were subjected to descriptive statistical calculations, and unless otherwise stated in the text and figure legends, data are shown as mean ± standard deviation (SD). Differences were considered statistically significant at a 95% level of confidence (α = 0.05).
3 RESULTS
3.1 Growth performance
The daily food intake and body weight gain of each group during the experimental period are summarized in Table 2. No significant differences were detected in these parameters among the four groups. However, both parameters decreased in the experimental groups compared with those of the control group.
| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | 50% | 25% | 0% | |
| Growth performance (n = 5 in each group) | ||||
| Daily food intake (g/day per chicken) | 70.0 ± 4.9 | 57.8 ± 13.3 | 68.9 ± 9.7 | 60.2 ± 7.8 |
| Body weight gain (g per chicken) | 127.3 ± 34.4 | 46.4 ± 63.1 | 79.5 ± 31.0 | 90.6 ± 20.8 |
| Ultrastructural features of enterocytes (n = 3 in each group) | ||||
| Length of microvilli (μm) | 1.81 ± 0.42a | 1.34 ± 0.47b | 1.31 ± 0.42b | 1.12 ± 0.35c |
| Width of microvilli (nm) | 112.62 ± 20.20a | 91.99 ± 19.74b | 83.00 ± 22.84c | 84.41 ± 22.18c |
| Linear density of microvilli (number/μm) | 9.40 ± 2.87a | 8.93 ± 1.37b | 7.57 ± 1.13c | 6.99 ± 1.38d |
| Terminal web thickness (μm) | 1.23 ± 0.31a | 1.00 ± 0.21b | 0.99 ± 0.36b | 0.83 ± 0.29c |
| Mitochondrial density (number/100 μm2) | 134.36 ± 53.74a | 108.16 ± 36.53b | 80.71 ± 28.65c | 70.11 ± 23.72c |
- Note: Values are expressed as mean ± standard deviation. Means not sharing the same subscript letters are significantly different at p < 0.05 (a, b, c, d).
3.2 Electron microscopy of enterocytes
The ultrastructural features of the enterocytes in the distal ileum from the control and 0% groups are shown in Figure 1. Obvious changes were detected in the enterocytes of the three experimental groups, especially in the 0% group compared with the control group. In the control group, the enterocytes had an ellipsoidal and electron-lucent nucleus with a smooth edge and contained mitochondria with clear cristae (Figure 1a). In contrast, many enterocytes in the 0% group showed a relatively electron-dense features and had a nucleus with a rough edge (Figure 1b). The mitochondrial cristae became tubular or unclear (Figure 1b,f). Moreover, there were obvious differences in the appearance of the microvilli between the control group and the 25% and 0% groups. Straight and tightly packed microvilli were observed in the control (Figure 1c,e) and 50% groups. In contrast, microvilli fragmentation was predominantly observed in the 25% and 0% groups (Figure 1b, arrows). Wide gap spaces were also observed between the microvilli in these groups (Figure 1d, arrows). Many small vacuoles with electron-dense and thick limiting membranes were contained in the apical cytoplasm of the enterocytes of the 25% and 0% groups (Figure 1b, asterisks).
The length, width, and liner density of the microvilli, terminal web thickness, and mitochondrial density were obtained by electron microscopy and expressed as means ± SD in Table 2. The control group showed the highest values of these ultrastructural feature parameters. There were significant differences in these parameters between the control and three experimental groups (p < 0.05). In contrast, the 0% group showed the lowest values of these ultrastructural feature parameters. Significant differences were also found in these parameters between the 0% and 50% groups (p < 0.05).
3.3 Immunoreactivity for villin and β-actin
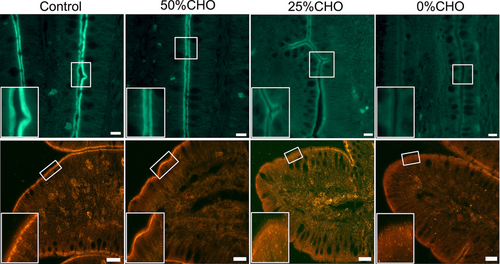
Villin and β-actin immunoreactivity was observed in the distal ileum of all groups. They were localized beneath the microvilli of the villous epithelium but not in the crypts (Figure 2, insets). No differences were found in the distribution pattern of villin and β-actin immunoreactivity among the four groups. However, the immunofluorescence intensity of villin was comparatively weaker in the 25% and 0% groups than in the control group (Figure 2, top panels). No obvious difference was observed in the immunofluorescence intensity of villin between the 25% and 0% groups. The immunofluorescence intensity of β-actin was also weaker in the 0% group compared with the other groups (Figure 2, bottom panels).
3.4 GLP-2-immunoreactive cells
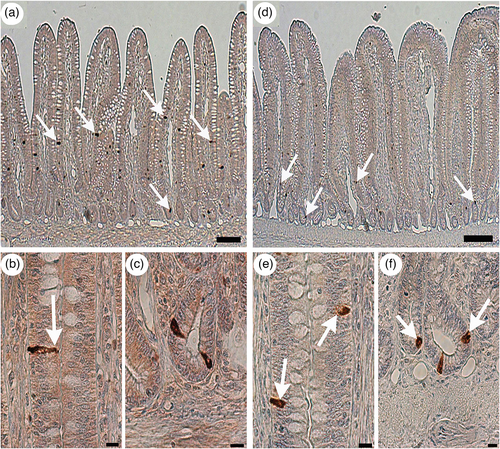
GLP-2-immunoreactive cells were frequently observed in the epithelium of the distal ileum for all groups. These were mainly localized in the middle part of the villi to the crypts (Figure 3a,d, arrows). These cells had a pyramidal- or spindle-like shape with a long cytoplasmic process in the villous epithelium (Figure 3b, arrow) and a comma-like shape in the crypts of the control group (Figure 3c). In contrast, oval- or round-shaped endocrine cells that showed GLP-2 immunoreactivity were frequently observed in the lower CHO-level groups, especially in the 0% group (Figure 3e,f, arrows). However, the distribution pattern of GLP-2-immunoreactive cells showed no obvious difference among the four groups.
Significant differences were detected in the frequency of GLP-2-immunoreactive cells among the four groups (Table 3). The frequency of GLP-2-immunoreactive cells was significantly higher in the control group than in the experimental groups. This amount decreased with the declining levels of dietary CHO and was lowest in the 0% group.
| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | 50% | 25% | 0% | |
| GLP-2 (cells/mm2) | 47.41 ± 17.54a | 35.41 ± 12.91b | 24.22 ± 7.40c | 17.36 ± 7.75d |
| PG mRNA (cells/mm2) | 6.41 ± 4.09a | 5.14 ± 3.21b | 1.62 ± 1.35c | 1.30 ± 1.20c |
- Note: Values are shown as mean ± standard deviation (n = 5 in each group). Means not sharing the same subscript letters are significantly different at p < 0.05 (a > b > c > d).
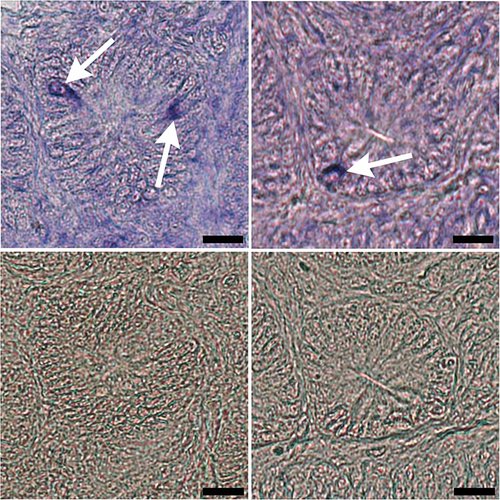
3.5 Cells expressing PG mRNA signals
PG mRNA signal-expressing cells were observed in the distal ileum of all groups (Figure 4). Cells that expressed PG mRNA signals were scattered in the epithelium of the bottom part of the villi and crypts. No obvious differences were observed in the distribution pattern of these cells among the four groups. There were significant differences in the frequency of cells that expressed PG mRNA signals among the four groups (Table 3). The 25% and 0% groups demonstrated considerably lower amounts than the control and 50% groups.
4 DISCUSSION
This study demonstrated that dietary CHOs contributed to the maintenance of microvilli in the chicken ileum and suggested that GLP-2 secretion from the intestinal L cells was involved in this maintenance system.
Microvilli are the main structure in the apical domain of enterocytes. These increase the apical free surface area for efficient absorption of ingested nutrients (Helander & Fändriks, 2014). This morphological adaptation allows the intestinal epithelium to have close and prolonged contact with luminal contents (Crawley et al., 2014). Consequently, the ingested nutrients can affect the structure of the enterocytes (Smith, 1992). Our previous study demonstrated that dietary CHOs induced a positive effect on the proliferation of enterocytes and goblet cells in the ileum epithelium of chickens (Salahuddin et al., 2021). In this study, the microvilli in the 25% and 0% groups showed obvious ultrastructure changes such as fragmentation and wide gaps between the microvilli. These findings provide evidence that microvilli fall down from the apical surface of enterocytes under CHO-free conditions. Morphometrical data obtained from electron microscopy demonstrated these ultrastructure changes in the microvilli, including shorter length, narrower width, and reduced linear density in the 25% and 0% groups compared with the control group. These results indicate that the low CHO diet reduced the surface area of the small intestine, which leads to a decrease in the efficiency of nutrient absorption. Terminal webs provide rigidity to the apex of enterocytes and an anchor for the brush border in the apical network of actomyosin and intermediate filaments (Delacour et al., 2016). This study indicates that the terminal webs were significantly thinner in the experimental groups than in the control group, which suggests that the embrittlement of the basement of the enterocyte microvilli became brittle. These ultrastructural features indicate that dietary CHOs are essential in maintaining the structure of the enterocyte microvilli.
Conspicuous structural alterations were observed in the enterocytes of the 25% and 0% groups, characterized by vacuoles with an electron-dense thick limiting membrane and signs of mitochondrial degeneration. Notably, the mitochondrial density in these groups was significantly lower compared with the control group. These findings align with the understanding that dietary CHOs are a primary energy source in poultry. The lack of CHOs may lead to a negative energy balance, potentially triggering mitochondrial degeneration and the formation of vacuoles in enterocytes. This observation resonates with the insights from Khaloian et al. (2020), who suggested that mitochondrial impairment, possibly due to a negative energy balance, can affect intestinal epithelial function by altering intestinal stem cell dynamics. Such hypothesis is also supported by the work of Smith (1992), emphasizing the importance of dietary nutrients in maintaining enterocyte structure and function. Additionally, Delacour et al. (2016) have corroborated the significant role of dietary factors in intestinal homeostasis. However, additional systematic analysis is required to understand this phenomenon.
Microvilli contain a cytoskeletal core that consists of approximately 20–30 actin filaments that are bundled by villin, ezrin, and fimbrin (Bretscher & Weber, 1980; Fath & Burgess, 1995). Villin is a major and versatile actin-binding protein that regulates biological functions in the enterocytes, such as cell morphology and actin dynamics (Khurana & George, 2008). This actin-binding protein is proposed to participate in the organization and stabilization of the brush border core bundles (Friederich et al., 1999). Drenckhahn and Dermietzel (1988) revealed that villin was found within the entire microvilli filament bundle in the chicken intestinal epithelium. Moreover, the cytoskeletal core proteins showed a quick turnover, thereby accounting for the stability of microvillar shapes in mature epithelial cells of chicken (Stidwill et al., 1984). In this study, immunohistochemistry demonstrated that β-actin and villin were localized just beneath the brush border of the chicken ileum in the control group. However, the immunofluorescence intensity of these structural proteins was weaker in the 0% group compared with the control group. Revenu et al. (2012) showed the alteration of microvilli structure in a mouse model that lacked actin-binding proteins including villin, but this change was minimal in a mouse model that only lacked villin. Similarly, this study revealed a lower β-actin immunoreactivity in the 0% group than in the control group. This finding indicated the ravel of the actin filament bundles in the microvilli. Based on these results, we propose that a CHO-free diet induced the reduction of villin and other actin-binding proteins, followed by the desquamation of the microvilli, which was observed through electron microscopy.
GLP-2 is derived from PG and is secreted from the intestinal L cells with a long cytoplasmic process that is in contact with the intestinal lumen. This peptide is involved in important physiological actions such as intestinal growth, crypt cell proliferation, and limiting epithelial apoptosis (Burrin et al., 2007; Drucker et al., 1996; Tsai et al., 1997). GLP-2-immunoreactive cells were mainly localized in the lower part of the villi and crypts of the distal ileum (Monir et al., 2014). GLP-2 and GLP-1 colocalized within the same secretory granule of L cells, which showed an open-type form of endocrine cells (Nishimura et al., 2013). Enteroendocrine cells in an open-type form are considered primary chemosensory agents that sense nutrients in the intestinal lumen (Breer et al., 2012) and secrete hormones in response to these ingested nutrients (Gribble & Reimann, 2016). Some studies have found that the ingestion of CHOs increases the secretion of GLP-2 in the mammalian small intestine (Rachmiel et al., 2019; Xiao et al., 1999). Notably, the frequency of GLP-2-immunoreactive cells in this study was significantly higher in the control group than in the experimental groups. A decreasing frequency of these cells was observed with decreasing levels of dietary CHO. Moreover, the lower CHO-level groups, especially the 0% group, showed many round- or oval-shaped GLP-2-immunoreactive cells. These cells were frequently found in the ileum epithelium obtained from fasted chickens and contained vacuoles in the perikaryon with small lobule nuclei (unpublished data). Similarly, results from this study indicated that GLP-2-immunoreactive cells found in the lower CHO-level dietary groups would eventually be eliminated from the epithelium due to the lack of sufficient amounts of CHO in the lumen. Low levels of CHO could be the main reason for the reduced frequency of GLP-2-immunoreactive cells in the lower CHO-level groups, especially in the 0% group.
In situ hybridization demonstrated that the frequency of cells that expressed PG mRNA signals in the 25% and 0% groups was significantly decreased compared with that in the control group. This finding suggests that dietary CHO influences the PG mRNA transcription level in the L cells of the chicken ileum. Previous studies have shown that the PG mRNA transcription level in L cell lines is upregulated when treated with higher glucose levels (Daoudi et al., 2011; Puddu et al., 2014). These studies strongly support this possibility. Therefore, it is possible that low levels of dietary CHO can impair PG mRNA transcription and reduce the frequency of GLP-2-containing cells in the distal ileum of chickens.
Another study demonstrated that multiple actions of GLP-2 on the intestinal epithelium were not directly targeted toward the crypt or epithelial cells, because GLP-2 receptors (GLP-2Rs) were not found on these cells (Dubé et al., 2006). GLP-2 activates its specific GLP-2R in the subepithelial intestinal myoblast cells that release insulin-like growth factor (IGF)-1. The released IGF-1 couples with the IGF-1 receptors (IGF-1Rs) of the crypt cells and stimulates their proliferation. Therefore, it was proposed that GLP-2 released in response to CHO ingestion stimulates the proliferation of enterocytes through the IGF-1 system in the chicken small intestine. Bulut et al. (2004, 2008) demonstrated that the effect of GLP-2 on intestinal epithelial cell repair is dependent on transforming growth factor-β. Similarly, Ørskov et al. (2005) revealed that the keratinocyte growth factor might be responsible for the intestinal proliferation that is induced by GLP-2. These growth factors from the intestinal subepithelial myoblast cells were proposed to be involved in this mechanism. However, further systematic studies are required to clarify this. In this study, corn oil was added as a source of fat at a higher inclusion level to maintain a constant energy level in the diets of the control and three treatment groups. The high fat levels had no significant effects on the results because dose-dependent changes induced by corn oil were not found in any of the experiments conducted in this study.
The microvilli of enterocytes are the front line of nutrient absorption and contain terminal digestive enzymes such as oligopeptidase and disaccharidase (Drozdowski & Thomson, 2006). In this study, we showed that a CHO-free diet causes morphological changes in the microvilli of enterocytes in the ileum, leading to a decrease in body weight, although the difference is not significant. This suggests that damage to the microvilli of the enterocytes may have resulted in inadequate nutrient absorption, with consequent body weight loss.
In conclusion, this study demonstrates that dietary CHOs are important not only for as a source of energy but also to maintain the structure of the enterocyte microvilli in the ileum of chickens. This maintenance system may depend on the secretion of GLP-2 and the stimulation of PG mRNA transcript levels in the intestinal L cells.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




