Ovarian dynamics in progesterone tablet-induced superovulation in goats assessed by magnetic resonance imaging
Abstract
Controlled internal drug-releasing (CIDR) devices are commonly used for superovulation in goats. However, such devices are unavailable in some countries, including Japan. In this technical note, we aimed to explore the efficacy of an alternative superovulation protocol using progesterone tablets in goats. We employed intravaginal progesterone tablets (LUTINAS® Vaginal Tablet 100 mg) following a standard superovulation protocol. Additionally, we assessed the ovarian dynamics using 3T-magnetic resonance imaging (MRI) 1 day preceding the progesterone treatment (Day “−1”) and 3 days before the end of treatment (Days 11–13). The ovarian monitoring was successfully performed in the short tau inversion recovery T2-weighted images of MRI, and ovulation was confirmed by the disappearance of follicles on Day 13 post-administration of the tablets. Immediately after ovulation, oviduct flushing yielded a substantial number of oocytes (13.5 ± 1.8 oocytes per animal). These findings provide evidence that the administration of progesterone tablets can serve as a viable alternative for inducing. Additionally, our findings suggest that 3T-MRI is a promising alternative to conventional ultrasonography for monitoring ovarian dynamics following superovulation in experimental goats.
1 INTRODUCTION
Goats, in particular, have garnered considerable attention as bioreactors for the production of therapeutic proteins within their mammary glands (Carneiro et al., 2018; Zhang et al., 2019). The collection of a large number of oocytes and zygotes is imperative, especially when aiming to harness transgenic technology for the development of such goats. Therefore, in goat breeding, the practice of advanced reproductive techniques, such as superovulation and subsequent embryo transfer, is important (Baldassarre et al., 2004; Baldassarre & Karatzas, 2004; Cognie, 1999). The popular protocols for superovulation in goats entail the use of FSH or eCG treatments in conjunction with the insertion of controlled internal drug-releasing devices (CIDR; e.g., CIDR® sheep/goat; Zoetis, Parsippany, NJ, USA) administered for 11–18 days (Amoah & Gelaye, 1990; Menchaca et al., 2010; Vilariño et al., 2011). However, in certain countries, including Japan, these devices are not readily available, and even their importation is restricted due to hygiene concerns. Therefore, identifying alternative methods to facilitate superovulation in goats in such countries is an urgent requirement.
Progesterone tablets are routinely used in obstetrics and gynecology and hold the potential to increase plasma progesterone levels, thereby regulating the function of the luteum during assisted reproductive technology (Shiba et al., 2020). These tablets serve the same purpose as CIDR or Sincrogest sponges in ruminant reproduction; therefore, they present a reliable alternative for enhancing goat breeding techniques.
Ultrasonography is the most commonly used diagnostic tool for monitoring ovarian dynamics (Adams, 1999). However, the precision of follicle counting, especially in veterinary practice, remains a challenge. Moreover, transrectal ovarian echography, frequently applied in large animal practice, does not translate well to smaller animals.
In the field of reproduction, magnetic resonance imaging (MRI) has been proven invaluable, serving not only as a diagnosis tool for tumors but also for observing normal ovarian structures (Nöthling et al., 2006). MRI successfully revealed hyperintense circular structures of the matured follicles within the abdominal cavity (Maeda et al., 2016), indicating its distinct advantages over traditional ultrasonography. Furthermore, MRI acquisition is not affected by the skill of the examiner and provides comprehensive visualization of the female reproductive organs. Although an MRI examination is time-consuming, it enables the precise quantification of follicles in some animals, particularly in cases where transrectal nor transabdominal ultrasonography poses challenges (Leonhardt et al., 2014; Maeda et al., 2016).
Therefore, in this study, we aimed to investigate the efficacy of human progesterone tablets (LUTINUS Vaginal Tablet 100 mg; Ferring Pharmaceuticals Co., Ltd., Tokyo, Japan) for induction of superovulation in goats. Additionally, we assessed the efficiency of 3T-MRI to monitor the ovarian dynamics in the progesterone tablets-treated goats to confirm ovarian dynamics in response to superovulation.
2 MATERIALS AND METHODS
2.1 Ethical statement
The animal experiments were approved by the Committee for Animal Research and Welfare of Gifu University (#2019-072) and were conducted in accordance with their guidelines. All goats were managed by a veterinarian throughout the experiments.
2.2 Animals
This study was conducted from October to December 2019 at the Field Science Center of Gifu University using four Shiba × Saanen crossbreed goats (n = 4, 3–5 years; average body weight: 27.1 ± 3.1 kg). Goats were housed in the indoor cage, fed with Sudan grass, Italian rye-grass silage, and formula feed, and provided free access to water.
2.3 Superovulation protocol
For superovulation, we used intravaginal progesterone tablets (LUTINAS® Vaginal Tablet 100 mg) instead of a progesterone device following the protocol described in previous studies (Batista et al., 2014; Knights & Singh-Knights, 2016). First, 2.0 mg of PGF2α (Veterinary Pronalgon F, Zoetis Japan) was intramuscularly injected, and then the progesterone tablet was inserted into the vagina with a gloved hand twice a day for the following 11 days. Forty-eight hours before the end of progesterone exposure (Day 9), a total of 20 AU FSH (Anthrin® 10; Kyoritsu Seiyaku, Tokyo, Japan), divided into six decreasing doses for 3 days (Days 9–11), was injected twice a day. Then, at the fifth administration of FSH on Day 11, which was approximately 12 h after the final progesterone treatment on Day 10, ovulation was induced by intramuscular injection of PGF2α (2.0 mg) and hCG (300 IU).
2.4 MRI acquisition
3T MRI (Achiveva 3.0T, Philips Japan, Tokyo, Japan) at the Animal Medical Center of Gifu University was used to monitor the follicular dynamics in goats. MRI acquisition was performed 1 day preceding the progesterone treatment (Day −1) and 3 days before the end of treatment (Days 11–13). For MR image acquisition, goats were sedated with intravenous administration of 2.0 mg/kg xylazine (2% Seractal®; Bayer, Osaka, Japan) and then anesthetized using 2%–5% isoflurane (IsoFlo; Bussan Animal Health Co., Ltd., Osaka, Japan). For the follicular imaging, we obtained pelvic multi-slice T1W TSE acquisitions in the transaxial (TR/TE = 695/12, FOV = 340 × 340 × 70, Matrix = 524 × 363; 20 slices with 0.5 mm slice thickness and without gaps, Voxel size = 0.65 × 0.93 × 3.00 mm3, NSA = 2, Angle = 60, TSE factor = 4, BW = 681.6 Hz, Rel. SNR = 1) and sagittal (TR/TE = 695/12, FOV = 340 × 340 × 104 mm, Matrix = 524 × 363, 30 slices with 0.5 mm slices thickness and without gaps, Voxel size = 0.65 × 0.93 × 3.00 mm3, NSA = 1, Angle = 60, TSE factor = 4, BW = 542.2 Hz, Rel. SNR = 1) planes, T2W SE CS2 acquisitions in the transaxial (TR/TE = 4013/100, FOV = 340 × 340 × 70 mm, Matrix = 564 × 380, 20 slices with 0.5 mm slices thickness and without gaps, Voxel size = 0.60 × 0.68 × 3.00 mm3, NSA = 2, Angle = 120, TSE factor = 23, BW = 434.6 Hz, Rel. SNR = 1) and sagittal (TR/TE = 5949/100, FOV = 340 × 340 × 104 mm, Matrix = 564 × 380, 30 slices with 0.5 mm slices thickness and without gaps, Voxel size = 0.60 × 0.68 × 3.00 mm3, NSA = 2, Angle = 120, TSE factor = 23, BW = 434.6 Hz, Rel. SNR = 1) planes, and short tau inversion recovery (STIR) T2W TSE acquisitions in the transaxial (TR/TE/TI = 5987/80/230, FOV = 340 × 340 × 70 mm, Matrix = 420 × 286, 20 slices with 0.5 mm slices thickness and without gaps, Voxel size = 0.81 × 1.01 × 3.00 mm3, NSA = 2, Angle = 120, TSE factor = 19, BW = 291.8 Hz, Rel. SNR = 1) and sagittal (TR/TE/TI = 8981/80/230, FOV = 340 × 340 × 104 mm, Matrix = 420 × 286, 30 slices with 0.5 mm slices thickness and without gaps, Voxel size = 0.81 × 1.01 × 3.00 mm3, NSA = 1, Angle = 120, TSE factor = 19, BW = 291.8 Hz, Rel. SNR = 1) planes. The acquired ovarian images were analyzed using OsiriX v 4.1.2 (Pixmeo Sàrl, Bernex, Switzerland) to measure the number of follicles.
2.5 Oocyte collection
Following the confirmation of ovulation through the observed reduction in the number of follicles in the MRI images, the goats were subjected to anesthesia with isoflurane for the laparotomy procedure. The oocytes were counted and observed using a stereomicroscope. Post-procedure, the goats underwent a recovery phase during which they received comprehensive care administered by a qualified veterinarian.
2.6 Statistics
The acquired images were analyzed using OsiriX software (Pixmeo Sàrl, Bernex, Switzerland) to measure the diameter and number of ovarian follicles. Data are shown as mean ± standard deviation.
3 RESULTS AND DISCUSSION
In response to the progesterone tablet treatment for the superovulation, 13.5 ± 1.8 oocytes per animal were successfully obtained by flushing the oviduct (Table 1 and Figure 1). This finding suggests the efficiency of our protocol to obtain an adequate number of oocytes (Baldassarre & Karatzas, 2004). Our findings show that despite it being time and labor-intensive, daily insertion of the progesterone tablets for 11 days can substitute progesterone devices for superovulation treatment in goats.
| Goat | Collected oocytes | Corpus hemorrhagicum | Number of follicles (left, right) | Ovulation rate (%) | |||
|---|---|---|---|---|---|---|---|
| Day −1 | Day 11 | Day 12 | Day 13 | ||||
| A | 10 | 44 | 0 (0, 0) | 23 (12, 11) | 23 (12, 13) | 12 (4, 8) | 47.8 |
| B | 11 | 43 | 2 (1, 1) | 26 (12, 14) | 28 (14, 14) | 11 (4, 7) | 60.7 |
| C | 17 | 23 | 5 (3, 2) | 28 (14, 14) | 34 (17, 17) | 12 (7, 5) | 64.7 |
| D | 16 | 14 | 7 (3, 4) | 13 (10, 3) | 20 (12, 8) | 9 (5, 4) | 55.0 |
| Mean ± S.E. | 13.5 ± 1.8 | 31.0 ± 7.4 | 3.5 ± 1.6 | 22.5 ± 3.3 | 26.3 ± 3.1 | 11.0 ± 0.7 | 57.1 ± 3.7 |
- Note: Ovulation rate (%) = (Day 12 − Day 13)/Day 12 × 100.

In response to the progesterone (P4) administration, the blood P4 concentration increased gradually to 70.2 ± 37.2 on Day 9 and then decreased to 32.6 ± 7.0 ng/mL on Day 13. This data showed that the progesterone tablet increased plasma progesterone levels to control the function of the luteum during the treatment.
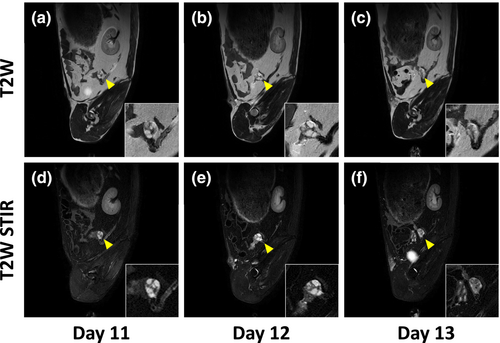
Next, we assessed the ovarian dynamics, follicular development, and ovulation in goats using MRI. The follicles were not visible in the T1-weighted image but were observed in the T2-weighted image. Moreover, in STIR T2 images, they were more prominent, displaying higher signals than the surrounding tissues (Figure 2). The detected follicular size was >3–7 mm, and the average number of follicles was 3.5 ± 1.6 on Day −1, increased to 22.5 ± 3.3 and 26.3 ± 3.1 on Days 11 and 12, respectively. The number of follicles decreased to 11.0 ± 0.7 on Day 13, and the ovulation was confirmed by the subsequent disappearance of follicles (57.1 ± 3.7% ovulation rate) on Day 13 (Table 1).
The procurement of oocytes and embryos plays an important role in breeding goats as livestock and laboratory animals. Therefore, more experimental techniques and tools in goat reproduction need to be developed. In this study, we showed that progesterone tablets used for assisted reproductive technology in humans could be an alternative to progesterone devices (such as CIDRs) for superovulation treatment in goats. The findings of this study demonstrated that MRI facilitates ovarian dynamics monitoring in small goats, where transrectal ultrasonography is challenging. Moreover, MRI is less dependent on the skill of the examiner and provides a wider observation field (Maeda et al., 2016). Therefore, these findings render MRI as a valuable tool for ovarian monitoring in small goats compared to ultrasonography.
Nonetheless, this study presents certain limitations. Our investigation exclusively employed the progesterone tablet for superovulation protocol, and we did not conduct a comparative analysis of ovarian responses between goats treated using CIDR devices as a control. Moreover, we did not evaluate the effects of sedation for MRI acquisition on oocyte numbers or quality. Therefore, to assert with confidence that the progesterone tablet can serve as a viable alternative to CIDR devices, further comprehensive studies are warranted.
In conclusion, this study demonstrates that progesterone tablets used for assisted reproductive technology in humans can serve as a viable alternative to devices like CIDR® sheep/goat for superovulation protocols and that MRI acquisition is an effective tool to monitor ovarian dynamics in small goats, despite being time- and labor-intensive.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest with respect to the publication of this manuscript.




