Fennel seeds dietary inclusion as a sustainable approach to reduce methane production and improve nutrient utilization and ruminal fermentation
Funding information: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript
Abstract
Ruminants are considered a major producer of methane (CH4). Therefore, the present study aimed to determine the ability of dry fennel seeds to affect in vitro gas production and fermentation. Fennel seeds were included at 0% (Control), 0.5%, 1%, 1.5%, and 2% DM of a diet containing per kg DM: 500 g concentrate feed mixture, 400 g berseem hay, and 100 g of rice straw. The incubations lasted 48 h. Fennel seeds increased (P < 0.001) the asymptotic gas production and decreased its rate, while decreasing the production and proportion of CH4 (P < 0.05) and increased its rate. Moreover, fennel seed increased DM and neutral detergent fiber (P < 0.01) degradability, and increased total production of short-chain fatty acids, acetate, and propionate (P < 0.05). Compared to the control, fennel seeds increased (P < 0.01) metabolizable energy, partitioning factor, and microbial crude protein production. Overall, fennel seeds can be included up to 2% DM in ruminant diets as an environmentally friendly product in animal farming due to its ability to improve feed utilization, ruminal fermentation and while reducing CH4 production.
1 INTRODUCTION
The main objective of some feed additives is to improve feed utilization, improve animal performance, and reduce methane (CH4) emissions. Phytogenic feed additives are plant-origin substances rich in bioactive plant compounds and have become alternatives to the use of synthetic or drug-based additives (Hegazy et al., 2023; Khattab et al., 2020; Matloup et al., 2017). Phytogenic and plant feed additives can modify ruminal fermentation patterns and ruminal microorganism activity (Kholif & Olafadehan, 2021). However, the effectiveness of these feed additives depends upon the source, type, and level of inclusion (Kholif & Olafadehan, 2021). The dose of the phytoconstituents is a premium issue that determines their effectiveness as feed additives for improving ruminal fermentation and digestion (Kholif et al., 2019; Salem et al., 2014). The administration of phytochemicals as a proxy to reduce CH4 emission from ruminants has been reported before (Ebeid et al., 2020).
Phytochemicals alter ruminal microbes and decrease methanogenesis (Kholif & Olafadehan, 2021). Phytochemicals can mitigate ruminal CH4 production through direct inhibition of methanogenic archaea and/or suppression of the microbial metabolic processes involved in methanogenesis (Kholif & Olafadehan, 2021). The antimicrobial activity (Bodas et al., 2012) of phytochemicals inhibits most ruminal microorganisms, especially methanogens (Bodas et al., 2012). The mode of action by which phytochemicals affect CH4 production differs from each other and the presence of terpenoids, phenolic, and phenols, are responsible for the CH4 reduction. Additionally, phytochemicals diminish the rate of CH4 production by altering archaeal communities, reducing methanogens activity, or inhibiting the growth of ruminal fungi and protozoa harboring methanogens (Kholif & Olafadehan, 2021).
Fennel (Foeniculum vulgare) is a plant rich in volatile components mainly estragole, eucalyptol and estragole, limonene, trans-anethole, α-pinene, fenchone, and fenchol (Moosavi-Zadeh et al., 2023). However, the concentrations of these components differ between types, strains, and geographic regions (Shalaby et al., 2011). Shalaby et al. (2011) showed that fennel from Egypt has high estragole and low anethole concentrations compared to other types of fennels from India, Germany, and the Netherlands fennel which contain high contents of anethole and low estragole. Due to these phytoconstituents, fennel has antioxidant, anti-inflammatory, and antimicrobial properties (Badgujar et al., 2014; Kargar et al., 2021; Nowroozinia et al., 2022). Mahmoud et al. (2020) and Fahim et al. (2022) fed lactating cows and buffaloes, respectively with Egyptian fennel and observed improved milk production and milk components. Recently, Moosavi-Zadeh et al. (2023) fed Holstein dairy cows on a diet supplemented with fennel from Iran at 25 or 50 g/d for 45 d and observed increased feed intake and milk yield and decreased ruminal acetate proportion. Kurniawati et al. (2020) showed that the inclusion of fennel essential oil from Indonesia at 25, 50, 75, and 100 μl/l of medium decreased dry matter (DM) degradability (DMD) and organic matter (OM) degradability (OMD). However, increased fiber degradability without affecting crude protein (CP) degradability compared to control. Moreover, Rahmy et al. (2019) included Egyptian fennel at 1%, 3%, 5%, and 7% of the incubated diet (roughage and concentrate ratio of 45:55) and observed that fennel decreased medium pH and ammonia-N and increased short-chain fatty acids (SCFA) without affecting nutrient degradability.
Until now, no scientific report is available focused on the use of Egyptian fennel included at different levels evaluating in vitro gas production, CH4 production, nutrient degradability, and fermentation kinetics in one study. Therefore, the objectives of the present study were to determine the effects of adding dried fennel seeds at different levels to a total mixed ration on in vitro gas production (GP), CH4 production, and in vitro ruminal fermentation. We hypothesized that the phytochemicals from dried fennel seeds would improve ruminal fermentation and nutrient degradability while reducing methane production.
2 MATERIALS AND METHODS
2.1 Ingredients and treatments
A basal total mixed ration (TMR) was prepared to be used as substrates and contained (per kg DM): 500 g concentrate feed mixture (CFM), 400 g berseem hay, and 100 g of rice straw. The CFM contained per kg of DM: 170 g of soybean seed meal, 395 g of wheat bran, 395 g of corn, 20 g of limestone, 10 g of vitamins and minerals mixture, and 10 g of salt. Nutrient contents of fennel and TMR are shown in Table 1.
| Fennel | CFMa | Berseem hay | Rice straw | Dietb | |
|---|---|---|---|---|---|
| Dry matter, g/kg fresh matter | 935.3 | 903.2 | 890.1 | 940 | 892.7 |
| Organic matter | 920.0 | 922.9 | 884.4 | 851 | 819.4 |
| Crude protein | 121.8 | 165 | 128.3 | 42 | 135.7 |
| Ether extract | 162.3 | 46.8 | 54.4 | 19 | 61.8 |
| Nonstructural carbohydrates | 253.6 | 414 | 224.2 | 166 | 359.1 |
| Neutral detergent fiber | 382.3 | 297.1 | 477.5 | 624 | 379.0 |
| Acid detergent fiber | 326.2 | 175.1 | 380.7 | 394 | 239.8 |
- a Concentrate feed mixture (CFM) contained per kg DM: 170 g soybean seed meal, 395 g wheat bran, 395 g maize, 20 g limestone, 10 g vitamins and minerals mixture, and 10 g salt.
- b Diets: Contained per kg DM: 500 g concentrate mixture, 400 g berseem hay, and 100 g rice straw.
Clean and dry Egyptian local fennel seeds (traditionally cultivated in Egypt for many years) were obtained from a local supplier in Egypt. Seeds were harvested in June 2021 after eight months of cultivation. Seeds were grounded through a 1-mm screen using a Wiley mill (Arthur H. Thomas, Philadelphia, PA, USA), and mixed before use. The volatile compounds in fennel seeds were measured using a GC system (model 7890B from Agilent Technologies, Colorado, USA) equipped with a flame ionization detector at Central Laboratories Network, National Research Centre, Egypt. Separation was achieved using a Zebron ZB-FAME column (60 m × 0.25 mm internal diameter × 0.25 μm film thickness). Analyses were carried out using hydrogen as the carrier gas at a flow rate of 1.8 ml/min at a split-1:50 mode, injection volume of 1 μl and the following temperature program: 100°C for 3 min; rising at 2.5°C/min to 240°C and held for 10 min. The injector and flame ionization detector were held at 250°C and 285°C, respectively.
2.2 In vitro fermentation and biodegradation
The study was conducted in accordance with the Declaration of Helsinki in accordance with the 3rd edition (2010) of the guide of Agricultural Research and Teaching of Federation of Animal Science Societies, Champaign, IL, USA. The protocol was designed and all practices were performed according to the Directives 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of the animals used for scientific purposes.
The in vitro fermentation medium was prepared according to Goering and Van Soest (1975). A reducing solution containing sodium sulfide was added (2 ml) to the buffer shortly before the rumen fluid addition. Ruminal inoculum (20 ml) and the buffer solution (80 ml) were mixed in each 250 ml bottle. Three separate incubation runs were performed. For each run, ruminal inoculum was collected from the rumen of three slaughtered Barki sheep (mature lambs) from a local slaughterhouse in Cairo (Egypt). Sheep were slaughtered according to the Egyptian and Islamic laws of animal slaughtering and carcass handling where any kind of stress or discomfort to the animals should be avoided, and animals must be handled gently with care. Before slaughtering, sheep were ad libitum fed a diet containing concentrate, (200 g of soybean seed meal, 400 g of wheat bran, 360 g of corn, 20 g of limestone, 10 g of vitamins and minerals mixture, and 10 g of salt) berseem hay and rice straw at 5:4:1 (DM basis), with free access to water. The ruminal fluid was filtered through two-layered- cheesecloth to remove large feed particles, and the particulate materials were squeezed to obtain microbes attached to feed particles. The initial pH of the inoculum was 6.5. All treatments were tested in three separate incubation runs with three replicates (bottles; analytical replicates) in each run for each treatment. In each incubation run, two bottles with inoculum but without feed (blanks) were also included to establish baseline fermentation gas production (GP).
A 1 g ± 10 mg sample of the formulated TMR was weighed into filter bags (ANKOM F57; Ankom Technology, Macedon, NY, USA) and then placed into 250 ml ANKOM bottles (AnkomRF Gas Production System) fitted with an automatic wireless in vitro GP module (Ankom Technology, Macedon, NY, USA) with pressure sensors. Fennel seeds were included at 0 (control), 0.5%, 1%, 1.5%, and 2% of the diet. The pressure was recorded every 10 minutes for 48 h, and cumulative pressure was calculated from these values. The gas pressure was converted into volume (mL) at standard pressure and temperature. The gas volume in the blank units was subtracted to obtain net GP. At each incubation time, gas samples (5 ml) were taken from the sampling vent and infused into a Gas-Pro detector (Gas Analyzer CROWCON Model Tetra3, Abingdon, UK) to measure the concentration of CH4.
2.3 Sampling and analysis of fermentation variables
After 48 h of incubation, the fermentation was stopped by placing the bottles on ice for 5 minutes, and then pH was measured immediately using a pH meter. The ANKOM F57 filter bags were dried in a forced air oven at 55°C for 48 h. Dry matter, neutral detergent fiber (NDF), and acid detergent fiber (ADF) degradation were calculated by subtracting the dried residue weight from the initial weight of the dried substrate. Total gas and CH4 production were expressed in relation to degraded DM, NDF, and ADF at 48 h of incubation.
Samples (5 ml) of the supernatant fermented fluid from each bottle were taken in glass tubes for determination of ammonia-N (NH3-N) and total and individual SCFA concentrations. A subsample of 3 ml was preserved with 3 ml of 0.2 M hydrochloric acid solution for measurement of NH3-N concentration according to AOAC (1997). An aliquot (0.8 ml) was mixed with 0.2 ml of metaphosphoric acid solution (250 g/l) for SCFA analysis by steam distillation and titration method.
2.4 Chemical analysis
Samples of fennel seed and TMR were analyzed for ash after burning the samples in a muffle furnace at 550°C for 12 h (method ID 942.05), CP using the Kjeldahl method (method ID 954.01), and ether extract (EE) using diethyl ether in Soxhlet extractors (method ID 920.39) according to AOAC (1997) methods. Neutral detergent fiber content was determined without alpha amylase but with sodium sulfite following the procedure of Van Soest et al. (1991). Acid detergent fiber content was analyzed according to AOAC (1997) (method ID 973.18) and expressed exclusive residual ash. Non-structural carbohydrate, cellulose, hemicellulose, and OM concentrations were calculated.
2.5 Calculations and statistical analyses
For the estimation of GP kinetic, total GP (mL/g DM) data were fitted using the NLIN procedure of SAS (Version 9.4, SAS Inst., Inc., Cary, NC) according to France et al. (2000) model as: y = A × [1 − e−c (t − Lag)] where y is the volume of total GP production at time t (h); A is the asymptotic GP (ml/g DM); c is the fractional rate of GP (/h), and Lag (h) is the discrete lag time before any GP.
Data were analyzed using the GLM procedure of SAS in a complete randomized design using the model: Yij = μ + Li + εij where: Yij is the observation, μ is the population mean, Li is the fennel level effect, and εij is the residual error. Data from each of the three runs of the same sample were averaged before statistical analysis. The mean values of each experimental run were calculated and used as the experimental unit. Linear and quadratic contrasts were employed to determine the responses to the administration of increasing levels of fennel. The comparisons among treatments were performed with Duncans multiple range test. Significance was declared at a level of P < 0.05.
3 RESULTS
3.1 Fennel
As shown in Table 2, fennel seeds contained 8 volatile compounds: estragole (62.1%), limonene (17.9%), L-fenchone (7.1%), α-pinene (3.8%), (E)-anethole (2.4%), myrcene (2.3%), β-pinene (1.3%), and 1,8-cineole (0.65%).
| Peak | Compounda | Concentration (%)b |
|---|---|---|
| 1 | Estragole | 62.1 |
| 2 | Limonene | 17.9 |
| 3 | L-fenchone | 7.12 |
| 4 | α-Pinene | 3.83 |
| 5 | (E)-anethole | 2.44 |
| 6 | Myrcene | 2.26 |
| 7 | β-Pinene | 1.29 |
| 8 | 1,8-Cineole | 0.65 |
- a Identification based on authentic standards, the National Institute of Standards and Technology (NIST) library spectra, and literature.
- b Concentration based on the total areas of the identified peaks.
3.2 Biogases production
Figures 1 and 2 show GP (mL) and CH4 production (mL), respectively per g DM, degraded DM, degraded NDF, and degraded ADF. The inclusion of fennel seed at all levels linearly increased (P < 0.001) the asymptotic GP and decreased the rate of GP (linearly at P < 0.001 and quadratically at P = 0.002) and the lag of GP (linearly at P = 0.006) (Table 3). Moreover, the inclusion of fennel seeds decreased the production (linearly at P = 0.01 and quadratically at P = 0.025) and proportion of CH4 (linearly at P = 0.002) and linearly increased the rate of CH4 (P = 0.01) and linearly increased the lag of CH4 production (P = 0.013).
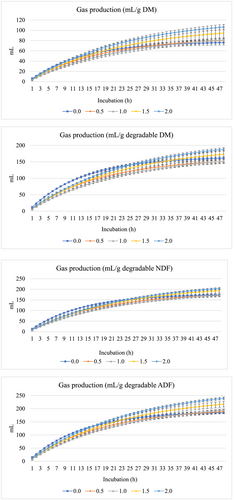
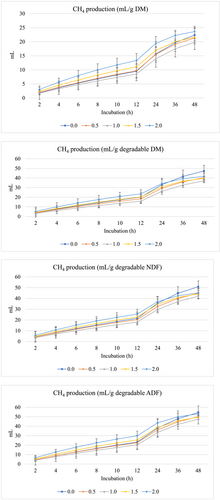
| GP parameters1 | CH4 parameters2 | ||||||
|---|---|---|---|---|---|---|---|
| Level | A | c | Lag | A | c | Lag | %3 |
| 0 | 78.1d | 0.076a | 1.55a | 28.6a | 0.035c | 1.52b | 29.3a |
| 0.5 | 86.5cd | 0.056b | 1.40ab | 25.7b | 0.040bc | 1.78a | 26.6ab |
| 1 | 92.7c | 0.043c | 1.20b | 24.4b | 0.036b | 1.78a | 24.5b |
| 1.5 | 108.2b | 0.044c | 1.22b | 23.6b | 0.053a | 1.76a | 23.0b |
| 2 | 124.0a | 0.041c | 1.23b | 25.0b | 0.064a | 1.86a | 22.2b |
| SEM | 3.66 | 0.003 | 0.075 | 0.92 | 0.0073 | 0.065 | 1.34 |
| P value | |||||||
| Treatment | <0.001 | <0.001 | 0.030 | 0.028 | 0.021 | 0.010 | 0.023 |
| Linear | <0.001 | <0.001 | 0.006 | 0.010 | 0.010 | 0.013 | 0.002 |
| Quadratic | 0.109 | 0.002 | 0.071 | 0.025 | 0.267 | 0.198 | 0.396 |
- Note: Means in the same row with different superscripts differ, P < 0.05. P value is the observed significance level of the F-test for treatment; SEM = standard error of the mean.
- 1 GP parameters: A is the asymptotic GP (ml/g DM), c is the rate of GP (/h), Lag is the initial delay before GP begins (h).
- 2 Methane (CH4) production parameters: A is the asymptotic CH4 production (ml/g DM), c is the rate of CH4 production (/h), Lag is the initial delay before CH4 production begins (h).
- 3 The proportional CH4 at the end of incubation (48).
3.3 Degradability and fermentation
Fennel seeds linearly increased DM (P = 0.001) and NDF (P < 0.001) degradability, without affecting ADF degradability (Table 4). Moreover, fennel inclusion increased the concentrations of total SCFA (linearly at P < 0.001 and quadratically at P = 0.01), acetate (linearly at P = 0.007 and quadratically at P = 0.017), and propionate (linearly at P = 0.003) without affecting those of butyrate. Without affecting fermentation pH, fennel seeds increased ME (linearly at P = 0.015 and quadratically at P = 0.023), PF24 (linearly at P = 0.022 and quadratically at P = 0.004), and MCP (linearly at P = 0.006 and quadratically at P = 0.009) compared to the control.
| Degradability1 | SCFA2 | Fermentation3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level | DM | NDF | ADF | Total | C2 | C3 | C4 | pH | NH3-N | ME | PF24 | MCP |
| 0 | 473b | 439c | 412 | 23.4c | 11.4b | 7.90b | 4.08 | 6.27 | 10.4 | 4.68ab | 7.22b | 328b |
| 0.5 | 533ab | 477bc | 425 | 26.0b | 13.2ab | 8.20a | 4.64 | 6.23 | 11.3 | 4.62ab | 8.46ab | 394a |
| 1 | 535a | 472b | 418 | 26.7ab | 13.6a | 9.56a | 3.56 | 6.33 | 11.0 | 4.52b | 8.97a | 404a |
| 1.5 | 546a | 489b | 438 | 27.0ab | 13.4a | 9.64a | 4.02 | 6.30 | 11.5 | 4.80ab | 7.83ab | 391a |
| 2 | 566a | 524a | 445 | 27.9a | 13.4a | 9.61a | 4.94 | 6.23 | 11.2 | 5.00a | 7.32ab | 396a |
| SEM | 13.1 | 7.4 | 12.8 | 0.32 | 0.38 | 0.391 | 0.235 | 0.039 | 0.29 | 0.089 | 0.368 | 12.0 |
| P value | ||||||||||||
| Treatment | 0.006 | 0.002 | 0.385 | <0.001 | 0.015 | 0.020 | 0.140 | 0.364 | 0.165 | 0.028 | 0.031 | 0.008 |
| Linear | 0.001 | <0.001 | 0.078 | <0.001 | 0.007 | 0.003 | 0.167 | 1.000 | 0.076 | 0.015 | 0.022 | 0.006 |
| Quadratic | 0.174 | 0.590 | 0.765 | 0.010 | 0.017 | 0.215 | 0.208 | 0.205 | 0.168 | 0.023 | 0.004 | 0.009 |
- Note: Means in the same row with different superscripts differ, P < 0.05. P value is the observed significance level of the F-test for treatment; SEM = standard error of the mean.
- 1 DM is dry matter degradability (g/g incubated), OM is organic matter degradability (g/g incubated), NDF is neutral detergent fiber degradability (g/g incubated), ADF is acid detergent fiber degradability (g/g incubated).
- 2 SCFA is short chain fatty acids (mmol/g DM), C2 is acetate (mmol/g DM), C3 is propionate (mmol/g DM), C4 is butyrate (mmol/g DM).
- 3 NH3-N is ammonia-N (mg/g DM), ME is metabolizable energy (MJ/kg DM), PF24 is the partitioning factor at 24 h of incubation (mg DMD: mL gas), MCP is microbial CP production (mg/g DM).
4 DISCUSSION
4.1 Fennel
Estragole (62.1%), limonene (17.9%), L-fenchone (7.1%), α-pinene (3.8%) and (E)-anethole (2.4%) are representative volatile compounds in fennel seeds. Shalaby et al. (2011) showed that the Egyptian fennel contains high estragole and low anethole concentrations compared to other types of fennel which contain high contents of anethole and low estragole. These volatile compounds have various biological activities, including anti-inflammatory, anti-allergic, antimicrobial, and antiviral effects (Korinek et al., 2021). In ruminants, feeding such additives causes changes in the rumen microbiome and fermentation kinetics (Kholif & Olafadehan, 2021). It is well recognized that the volatile compounds in plant seeds have strong antimicrobial effects against ruminal microbiota (i.e., fungi, protozoa, and bacteria), due to the phenolic and non-phenolic compounds. These phenolic and non-phenolic compounds have the potential to disintegrate bacterial cell membranes allowing ion leakage (Kholif & Olafadehan, 2021). Due to their high levels of volatile compounds, phytogenic feed additives have strong antimicrobial properties on ruminal protozoa, fungi, and bacterial populations (Newbold et al., 1997). These volatile compounds can suppress readily fermentable substrate digestibility, affecting fiber digestion (Kim et al., 2019). Patra et al. (2010) observed that fennel decreased carboxymethyl-cellulase and xylanase activities. Collectively, such effects on ruminal microbes produce some changes in ruminal fermentation kinetics, and this will be discussed later.
4.2 Gas production
Gas production is generally used as an indicator for digestibility, fermentability, and microbial protein production. In the present study, fennel seed linearly increased the asymptotic GP, indicating enhanced ruminal fermentation due to the presence of phytochemicals in the seeds. Phytochemicals favorably increase GP at appropriate levels (Salem et al., 2014). At appropriate levels, phytochemicals in fennels may suppress protozoal populations (Hegazy et al., 2023), and this could lead to increases in bacterial and fungal populations (Kholif, 2023; Kholif & Olafadehan, 2021).
Fennel seed administration linearly decreased the rate and lag of GP, which confirms the inverse relationship between the asymptotic GP and the rate of GP (Elghandour et al., 2015). The lowered lag of GP indicates the ability of rumen microorganisms to degrade fennel phytochemicals and utilize them as an energy source (Kholif & Olafadehan, 2021). In the present study, all levels showed positive effects on GP parameters, indicating that the evaluated levels of fennel seeds were within the range where ruminal microbes (i.e., bacteria, protozoa, and fungi) are capable of degrading the phytochemicals from fennel seeds.
4.3 Methane production
It was expected that improving the fermentability of the incubated diet with fennel seeds administration would decrease CH4 production. However, the inclusion of fennel seeds decreased the production and proportion of CH4 and increased the rate of CH4, indicating that fennel administration in ruminant diets can be used as a sustainable approach to inhibit methanogenic activity and mitigate CH4 production. It has been reported that ethanol and methanol extracts from fennel potentially inhibit rumen methanogenesis without adversely affecting rumen fermentation (Patra et al., 2010).
The presence of secondary metabolites in the fennel seeds inhibits the activity of methanogens and decreases ruminal CH4 production (Ku-Vera et al., 2020). Moreover, the phenols in fennel seeds have strong antibacterial effects on some microbial species such as Staphylococcus aureus, Escherichia coli, and Salmonella typhi (Peixoto et al., 2011), and on CH4-producing archaea in the rumen (Kholif & Olafadehan, 2021; Ku-Vera et al., 2020). Moreover, phenolic compounds decrease CH4 production via the inhibition of methanogen growth and decreasing interspecies H2 transfer (Ku-Vera et al., 2020). Hydrogen that is not used for CH4 production will be used in the synthesis of SCFA (Ungerfeld, 2020).
Fennel seeds increased the lag of CH4 production, which is an indicator of antimethanogenic activity. The delay in the onset of CH4 and thus a longer lag time suggests that fennel delayed methanogenic archaea and bacteria adaptation to fennel. In the rumen, H2 is produced from carbohydrates and amino acids fermentation and can be in two forms: dissolved or gas. The dissolved H2 is used by methanogens as a major energy source and later converted into CH4, but gas H2 is not used and only the dissolved H2 is biologically available for microbial growth. The rest of the H2 escapes from the rumen in a liquid phase (Wang et al., 2014).
4.4 Degradability and fermentation
Ruminal fermentation is sensitive to the presence of phytochemicals in the additives (El-Zaiat et al., 2020). It was expected that the inclusion of fennel would decrease ruminal pH, however, this was not observed in the present study. Fennel seeds increase nutrient digestibility and fermentation rate and fermentation byproducts, leading to a ruminal pH decrease (Mahmoud et al., 2020; Moosavi-Zadeh et al., 2023). Hegazy et al. (2023) reported unchanged ruminal pH in sheep-fed fennel at 1.2 g/kg DM. Fennel seeds administration did not affect ruminal NH3-N concentration. However, values were within the normal range (8.5 to over 30 mg ammonia N/dL) for optimal microbial growth and activity (Jones & Jones, 2012). Similar results were observed in many studies (Moosavi-Zadeh et al., 2023).
Fennel inclusion increased the concentrations of total SCFA, acetate, and propionate indicating that there was an enhanced carbohydrate fermentation. In this regard, Moosavi-Zadeh et al. (2023) and Hegazy et al. (2023) showed that animal-fed fennel increased propionate and total SCFA production. The phytoconstituents in fennel seeds can interact with microbial cell membranes and inhibit the growth of some gram-positive and gram-negative bacteria, resulting in lower acetate and higher propionate concentrations (Kholif & Olafadehan, 2021). The increased total and individual SCFA are indications of improved diet fermentability because SCFA is the main end product of ruminal carbohydrate fermentation. In the present study, fennel seeds improved DM and NDF degradability, which may explain the results obtained from SCFA. Plant secondary metabolites such as estragole, limonene, and L-fenchone, induce ruminal bacteria to degrade structural and nonstructural carbohydrates to reduce acetate and increase propionate production (Kholif, 2023; Patra & Saxena, 2010). Acetate and butyrate promote CH4 production while propionate formation can be considered as a competitive pathway for H2 use in the rumen (Karekar & Ahring, 2023).
Increasing NDF digestibility is beneficial for rumen fiber digestion (Jouany & Morgavi, 2007). Secondary metabolites and antioxidant properties of fennel seeds affect ruminal fibrolytic microbes and microbial growth and this was observed in the present study, as fennel seed increased DM and NDF degradability (Singla et al., 2021) resulting in faster degradation rate and extent of substrates (Kholif & Olafadehan, 2021). Phytochemicals and phenolics in the seeds may be considered the main reason for these changes due to their ability to stimulate microbial growth and activity within the rumen to degrade cell-wall constituents (Kholif & Olafadehan, 2021).
Secondary metabolites (e.g., Estragole, limonene, L-fenchone, etc.) in fennel and many other herbal plants have been reported to stimulate fibrolytic microbial activities in the rumen (Morgavi et al., 2000) which increase the rate of fermentation and degradation of substrates (Kholif & Olafadehan, 2021). Ebeid et al. (2020) showed that rumen microbiota can use plant secondary metabolites (e.g., phenolics and essential oils) and utilize them as energy sources (Kholif & Olafadehan, 2021). Moreover, the antiprotozoal effects of the phytochemicals in fennel (Hegazy et al., 2023) may be another reason. The secondary metabolites in fennel are lipophilic and can enter across the protozoal membrane and cause destruction of the cell membrane and inhibition of enzymes required for cell metabolism (Goel et al., 2005). Similar results were observed by Kurniawati et al. (2020) when fennel essential oil at 75 μl/l in vitro medium. Hegazy et al. (2023) showed that fennel administration at 1.2 g/kg DM increased in vitro DMD and OMD, and DM, OM, and CP digestibility in sheep. Mahmoud et al. (2020) noted improved nutrient digestibility with fennel seed administration to lactating cows.
Fennel seeds increased ME, PF24, and MCP, indicating improved synchronization between energy and protein release in the rumen, resulting in higher microbial protein synthesis and PF24. Phenolic compounds in fennel may interact with biosynthesis of aromatic amino acids, as both synthesis pathways are linked through phytochemicals from the seeds (Mandal et al., 2010). Results from increased MCP indicated that most NH3-N and SCFA were used for MCP synthesis (Boucher et al., 2007).
5 CONCLUSIONS
Overall, fennel seeds shifted rumen fermentation towards a more efficient utilization of dietary nutrients. The addition of fennel seeds at 0.5 to 2% DM improved rumen fermentation kinetics. Fennel seeds showed positive effects on GP and nutrient degradability while lowering CH4 production, and therefore fennel seeds could be used as an environmentally friendly way for livestock feeding. Further in vivo trials should be conducted to analyze rumen microbiome changes.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.




