Investigation of contributors to zinc protoporphyrin IX formation at optimum pH 5.5 in pork
Abstract
We investigated zinc protoporphyrin (ZnPP) formation in pork at pH 5.5, identified the contributors to ZnPP formation, and verified the involvement of myoglobin in this process. When pork homogenate was separated into two water-soluble fractions (>10 and <10 kDa) and an insoluble fraction, ZnPP formation was suppressed. ZnPP formation was rescued after mixing of all three fractions. Heating of the soluble <10 kDa fraction did not suppress the formation of ZnPP as opposed to heating of the soluble >10 kDa fraction, suggesting that protein(s) presents in the >10 kDa fraction contributed to ZnPP formation. Components of the soluble 10–30 kDa fractions separated by ultrafiltration were important in ZnPP formation. Exogenous myoglobin was not essential for ZnPP formation. A gel filtration study showed that soluble protein(s) with molecular weight higher than that of myoglobin was involved. Therefore, it was suggested that the soluble <10 kDa fraction, the insoluble fraction, and the soluble 10–30 kDa fraction (excluding myoglobin) are essential for ZnPP formation in pork at pH 5.5.
1 INTRODUCTION
Zinc protoporphyrin IX (ZnPP) is considered to be an important natural red pigment in dry-cured or dry-fermented meat products without nitrate or nitrite (Wakamatsu, Nishimura, & Hattori, 2004). Researchers have studied the mechanism of the formation of ZnPP in meat for several years. Elucidation of the ZnPP formation mechanism will be helpful in improving the color of meat products without addition of nitrate or nitrite. Although not completely elucidated, three possible mechanisms have been suggested for the formation of the red pigment in meat products: (a) a nonenzymatic reaction in which ZnPP is formed under anaerobic conditions (Becker, Westermann, Hansson, & Skibsted, 2012); (b) enzymatic reactions where ferrochelatase (FECH) is directly involved (Becker et al., 2012; Chau, Ishigaki, Kataoka, & Taketani, 2011; Wakamatsu, Okui, Hayashi, Nishimura, & Hattori, 2007); and (c) bacterial enzymatic reactions (Morita, Niu, Sakata, & Nagata, 1996). Because there are few microorganisms inside dry-cured ham, the third (bacterial) mechanism is believed to play a minor role in the formation of ZnPP in pork and dry-cured ham.
ZnPP was first proposed to be formed from myoglobin by a transmetallation process in which iron (II) ion is substituted by zinc (II) (Wakamatsu, Nishimura, et al., 2004; Wakamatsu, Okui, Ikeda, Nishimura, & Hattori, 2004). Myoglobin is a major heme protein in meat and has been considered as a heme donor in the formation of ZnPP (Wakamatsu, Nishimura, et al., 2004). The heme iron of myoglobin present in postmortem muscle tissue suggests that the substitution of iron by zinc occurs in ZnPP formation (Adamsen, Møller, Parolari, Gabba, & Skibsted, 2006). Chau et al. (2011) reported that exogenous recombinant yeast FECH facilitates the production of ZnPP from myoglobin-heme and heme in meat via the replacement of iron in the protoporphyrin ring by zinc ions. FECH catalyzes the insertion of Fe2+ into PPIX (protoporphyrin IX) in a living body but inserts other divalent metals such as zinc into porphyrins (Chau, Ishigaki, Kataoka, & Taketani, 2010; Taketani et al., 2007). On the other hand, it has been reported that ZnPP is not formed from heme (Wakamatsu et al., 2007). In addition, because the molecular weight of FECH (42 kDa) is considerably greater than that of myoglobin (17 kDa), it is suspected that it would be difficult for FECH to assist the de-ironing of heme located in the globin pocket of myoglobin.
ZnPP is formed in pork homogenates by both enzymatic and nonenzymatic reactions (Becker et al., 2012) and the amount is proportional to the Zn content in dry-cured meat products (Adamsen, Møller, Laursen, Olsen, & Skibsted, 2006). FECH is located at the inner membrane of the mitochondria in mammalian cells (Taketani, 1993) and hence is relatively insoluble. On the other hand, myoglobin is distributed throughout the cytoplasm (Ordway, 2004) and is a water-soluble component. Although it is presumed that Zn, FECH, and a heme donor such as myoglobin play important roles in ZnPP formation, the exact mechanism is not well known. Zinc in meat is believed to exist as the free ion or in complex with various zinc-binding proteins; the zinc source for ZnPP formation is still unclear. FECH and myoglobin are in different locations in muscle; hence, separation of fractions is important to elucidate their role in ZnPP formation. To the best of our knowledge, there are no studies until now on the contributors to ZnPP formation after separation of pork homogenate fractions.
The pH may influence enzymatic activity in biological processes. The pH optima of the FECH activity ranges from 5.5 to 6.0 for iron removal and from 7.5 to 8.0 for zinc insertion as investigated using an in vitro model with porcine mitochondria (Chau et al., 2010), whereas the pH in raw meats is 5.5–6.0 (Adamsen, Hansen, Møller, & Skibsted, 2003; Adamsen, Møller, Hismani, & Skibsted, 2004). The pH of meat is considered as an important factor for ZnPP formation. The amount of ZnPP peaked at pH 5.5 and decreased considerably at lower or higher pH (Wakamatsu et al., 2007). However, it is unclear how FECH would facilitate ZnPP formation at pH 5.5, as it requires higher pH for its Zn insertion activity.
We hypothesized that multiple components from pork homogenate contribute to ZnPP formation at pH 5.5. Therefore, we fractionized pork homogenate by centrifugation and checked the ZnPP-forming ability of the separated fractions. Furthermore, we separated the water-soluble fraction of pork homogenate by gel filtration chromatography and ultrafiltration to check the ZnPP-forming ability of the separated soluble fractions. Thus, the aim of this study was to find the contributors to ZnPP formation and verify the involvement of myoglobin in this process at an optimum pH of 5.5 in pork.
2 MATERIALS AND METHODS
2.1 Materials
Since ZnPP formation was optimum at pH 5.5 in longissimus dorsi muscle (Wakamatsu et al., 2007), longissimus dorsi muscle was used in this section. Porcine longissimus dorsi muscle was collected in triplicate from three primal cuts of common pigs produced in Hokkaido, Japan. After removing fat and connective tissue, the muscle was minced, packaged, and stored frozen (−20°C) until use.
2.2 Fractionation of pork
Pork (20%) was homogenized with pure water at 10,000 rpm for 90 s using a homogenizer (CELL MASTER CM-100; ASONE, Tokyo, Japan). Pork homogenate was adjusted to pH 5.5 and centrifuged (39,920 × g, himac CR20F; Hitachi Koki, Tokyo, Japan) for 20 min at 4°C. The supernatant was filtered through filter paper (No. 2, Toyo Roshi, Japan) to yield the filtrate as the water-soluble fraction. The precipitate was diluted with pure water up to the initial volume and then homogenized and centrifuged in the same manner as before for two more times to completely remove the water-soluble fractions. The precipitate was homogenated with pure water up to the initial volume to yield the insoluble fraction. The water-soluble fraction was dispensed into an ultrafiltration tube (VIVASPIN 20, 10,000 MWCO PES, VS1501; Sartorius Stedim Lab Ltd., Stonehouse, UK) through a sterile syringe filter (Minisart® syringe filter, 0.45 μm, Sartorius Stedim Biotech GmbH). It was then separated by centrifugation (8,000 × g, 90 min, 4°C) to obtain the >10 kDa soluble fraction and the <10 kDa soluble fraction. After separation, the >10 and <10 kDa soluble fractions were heat-treated separately using a water bath for 30 min at 100°C and cooled before applying to the model system.
2.3 ZnPP formation model experiment system
After fractionation of pork homogenate, individual and mixed fractions were applied to the ZnPP formation model experiment system as shown in Table 1. Antibiotics were added to the model samples to final concentrations of 70 μg/ml penicillin G potassium, 250 μg/ml streptomycin sulfate, and 50 μg/ml gentamicin sulfate. The solutions were kept in gas-impermeable bags and incubated at 25°C for 5 days in the dark. Anaerobic conditions were achieved and verified by using an oxygen absorber (A-500HS; I.S.O., Yokohama, Japan) and oxygen indicator tablets, respectively.
| Homogenate | Precipitate (insoluble fraction) | Supernatant (soluble fraction) | >10 kDa soluble fraction | <10 kDa soluble fraction | <10 kDa soluble fraction (heated) | >10 kDa soluble fraction (heated) |
|---|---|---|---|---|---|---|
| 0.5 ml | — | — | — | — | — | — |
| — | 0.25 ml | — | — | — | — | — |
| — | — | 0.5 ml | — | — | — | — |
| — | 0.25 ml | 0.5 ml | — | — | — | — |
| — | 0.25 ml | — | 0.5 ml | — | — | — |
| — | 0.25 ml | — | — | 0.5 ml | — | — |
| — | 0.25 ml | — | 0.5 ml | 0.5 ml | — | — |
| — | 0.25 ml | — | 0.5 ml | — | 0.5 ml | — |
| — | 0.25 ml | — | — | 0.5 ml | — | 0.5 ml |
- After addition of antibiotics and the indicated volumes of the respective fractions, the final volume of each sample was made up to 1.5 ml with pure water.
2.4 Fluorescence analysis
Acetone extraction and fluorescence analysis were performed as previously described (Wakamatsu, Okui, et al., 2004). After extraction with acetone, the fluorescence intensity of the extract was measured using a spectrofluorophotometer (RF-5300PC; Shimadzu, Kyoto, Japan). Fluorescence intensity at 590 nm for excitation at 420 nm was taken as a measure of the amount of ZnPP formed. All operations were carried out in the dark as far as possible.
2.5 Separation of water-soluble fraction according to molecular weight
The water-soluble fraction was subjected to ultrafiltration through various molecular weight cutoff ultrafiltration tubes (10, 30, 50, and 100 kDa; VIVASPIN 6, VS06S1; Sartorius Stedim Lab). Separated molecular weight fractions (filtrate) were applied to the ZnPP formation model experiment in two groups. One group was without the addition of the <10 kDa soluble fraction and another group was with the addition of the <10 kDa soluble fraction. After incubation in the presence of antibiotics, the formed ZnPP was evaluated as described above.
2.6 Addition of exogenous myoglobin to the ZnPP formation model experiment
Myoglobin (horse muscle-derived, Nacalai Tesque Inc., Kyoto, Japan) and sodium ascorbate (Kanto Chemical, Tokyo, Japan) were dissolved in pure water to 0.1 and 0.4 mg/ml, respectively, for the preparation of oxymyoglobin solution. Oxymyoglobin was dialyzed overnight with pure water to remove the low molecular weight impurities to obtain desalted oxymyoglobin. Oxymyoglobin and desalted oxymyoglobin were added in the model solution to the final concentration of 0.01%, 0.02%, and 0.05%. These two types of oxymyoglobin solution were added into pork homogenate or the mixture of the insoluble fraction and the <10 kDa soluble fraction. After incubation, the formed ZnPP was evaluated as described above.
2.7 Separation of water-soluble proteins by gel filtration chromatography
The water-soluble fraction (15 ml) of pork homogenate was concentrated using an ultrafiltration tube (VIVASPIN 15, 10,000 MWCO PES, VS1501; Sartorius Stedim Lab) by centrifugation (8,000 × g, 90 min, 4°C, himac CR20F; Hitachi Koki). The concentrated >10 kDa soluble fraction (about 1 ml) was applied to Toyopearl HW 50 gel filtration column (2.5 φ × 50 cm + 1.5 φ × 120 cm; Tosoh Co., Tokyo, Japan). The mobile phase consisted of 50 mmol/L sodium chloride and 10 mmol/L citrate buffer (pH 5.5). Flow rate was 12 ml/hr. Eluted fractions were collected in test tubes with a fraction collector, with test tubes being changed every 30 min (6 ml/test tube). The absorbance of the collected fractions was measured at 280 nm for protein content and at 412 nm for myoglobin content using a spectrophotometer (UV-1800; Shimadzu, Co., Kyoto, Japan).
2.8 SDS-PAGE
SDS-PAGE was performed with a polyacrylamide (AA) gel (4.5% AA in stacking gel and 15% AA in separating gel) according to conventional methods. The gel was applied to the electrophoresis apparatus (AE-6530P; ATTO, Co, Tokyo, Japan) with SDS-PAGE electrophoresis buffer (25 mmol/L Tris, 192 mmol/L glycine, 0.1% SDS) and stained overnight with coomassie brilliant blue.
2.9 Statistical analysis
Measurements were expressed as means ± SE. Each experiment conducted for three different times. Statistical analyses were performed using Microsoft Excel 2007 with Ekuseru-Toukei 2006 (Social Survey Research Information, Tokyo, Japan) as add-in software. Differences among the individual conditions were analyzed by using one-way analysis of variance with the multiple comparison tests of Tukey. A probability of p < 0.05 was considered statistically significant. For gel filtration chromatography data, ZnPP was expressed as the average values from duplicates for each of the fractions.
3 RESULTS AND DISCUSSION
3.1 Effect of fractionation of pork on ZnPP formation
To find the contributors to ZnPP formation, we fractionized pork homogenate into different fractions and investigated the effect of different pork fractions on ZnPP formation (Figure 1). When pork homogenate was fractionated into soluble and insoluble fractions and incubated separately, ZnPP formation was significantly suppressed. When they were mixed together, ZnPP formation was rescued. When the soluble fraction was further fractionated into the >10 and <10 kDa soluble fractions and incubated with insoluble fraction separately, ZnPP formation was significantly suppressed (Figure 1). When the separated soluble fractions were mixed together with insoluble fraction, ZnPP formation was rescued to a level similar to pork homogenate. Because FECH, considered to be involved in the formation of ZnPP (Becker et al., 2012; Benedini, Raja, & Parolari, 2008; Parolari, Benedini, & Toscani, 2009; Wakamatsu et al., 2007), is the terminal enzyme of the heme-biosynthetic pathway in mitochondria, it is likely to be present in the insoluble fraction. In addition, some other insoluble components present in mitochondria, such as protoporphyrinogen oxidases, and protoporphyrinogen IX, are components in the heme-biosynthetic pathway (Koch et al., 2004; Poulson, 1976) and might have a role in ZnPP formation. In this study, we found that two water-soluble fractions (>10 and <10 kDa) and insoluble fractions of pork homogenate are essential components in a model system for ZnPP formation. Therefore, three or more components from these three separate fractions may have a combined role in ZnPP formation at pH 5.5.

3.2 Effect of heat treatment of fractionated water-soluble fractions on ZnPP formation
Hamm and Deatherage (1960) reported that about 77% of the water-soluble globular proteins of the bovine sarcoplasm are denatured by heating at 60°C for 30 min. Therefore, we examined the effect of heating on the >10 kDa soluble fraction to investigate the contribution of proteins presents in that fraction (Figure 1). When the heated >10 kDa soluble fraction was incubated with the insoluble fraction, ZnPP formation was significantly inhibited. Water-soluble fractions of pork homogenate contain many water-soluble sarcoplasmic proteins and most of the sarcoplasmic proteins are present in the >10 kDa soluble fraction. Myoglobin is the sarcoplasmic heme protein primarily responsible for the color of meat (Livingston & Brown, 1981), and according to its molecular weight, would be present in the >10 kDa soluble fraction. Okayama, Fujii, and Yamanoue (1991) reported that the percentage of denaturation of sarcoplasmic proteins was enhanced by a heating temperature in the range of 50–80°C. The observations in this study are consistent with the denaturation of proteins presents in the >10 kDa soluble fractions due to heating, resulting in the suppression of ZnPP formation. Therefore, this study suggested that water-soluble protein(s) present in the >10 kDa soluble fraction contribute to ZnPP formation at pH 5.5.
Next, we examined the effect of heating of the <10 kDa soluble fraction on ZnPP formation (Figure 1). We found that when the heated <10 kDa soluble fraction was incubated with the insoluble fraction, ZnPP formation was not suppressed to the extent seen for the corresponding experiment with heated >10 kDa fraction. There are many low molecular weight components, such as amino acids, vitamin B1, pantothenic acid, zinc ion, and iron ion, that are present in the <10 kDa soluble fractions of pork homogenate. Among them, vitamin B1 and pantothenic acid are heat-sensitive. Therefore, we suggest that such heat-sensitive compounds do not contribute to ZnPP formation. Adamsen, Møller, Laursen, et al. (2006) found a positive correlation of both Zn and Fe content to the logarithmic-transformed ZnPP content. Benedini et al. (2008) reported that a mixture of Zn2+ and PPIX forms ZnPP in the presence of meat extract. If zinc ion from the <10 kDa fraction is a contributor, mixing of the insoluble fraction and the >10 kDa soluble fraction with EDTA will increase PPIX formation. Although we examined the effect of fractionation of pork on PPIX formation with the addition of EDTA, mixing of the heated <10 kDa soluble fraction with the insoluble fraction and the >10 kDa soluble fraction did not rescue the formation of PPIX (data not shown). We speculated that heat-resistant components other than zinc ions presents in the <10 kDa soluble fraction might contribute to ZnPP formation. Further investigations are needed to identity the <10 kDa soluble component contributing to ZnPP formation.
3.3 Effect of separation of water-soluble fraction according to molecular weight on ZnPP formation
In order to examine the contribution of the water-soluble proteins to ZnPP formation, we investigated the molecular weight of the water-soluble contributor to ZnPP formation. The water-soluble fraction of pork homogenate was separated by ultrafiltration according to a molecular weight cutoff of 10–100 kDa and their ZnPP-forming abilities were measured (Figure 2a). After separation of <10, <30, <50, and <100 kDa soluble fractions, the separated fractions were incubated separately with the insoluble fraction. The results showed that ZnPP formation was significantly lower in the <10 kDa soluble fraction as compared to the control or to any of the other (<30, <50, and <100 kDa) soluble fractions. ZnPP formation was increased in the <30 kDa soluble fraction and remained at the same levels in the <50 and <100 kDa soluble fractions. The sarcoplasmic proteins are a mixture of several hundred molecular species, the complexity of which has been shown by modern proteomic techniques such as two-dimensional electrophoresis (Bendixen, 2005). The sarcoplasmic proteome is constituted by some soluble proteins including myoglobin and enzymes which can participate in different biochemical processes that may exert an impact on the stability of meat color. Grossi, do Nascimento, Cardoso, and Skibsted (2014) reported that proteolytic degradation of myoglobin and precipitation of inorganic iron in the meat matrix are essential for the metal exchange to form ZnPP and non-heme iron species. Therefore, our present study reveals that water-soluble proteins having molecular weights above 10 kDa and less than 30 kDa contribute to ZnPP formation at pH 5.5.
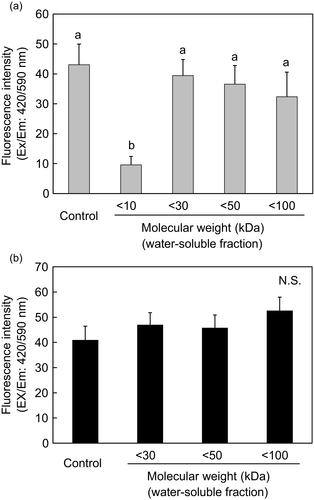
Further, we observed the supplemental effect of the <10 kDa soluble fraction on ZnPP formation in separated molecular weight groups (Figure 2b). When the separated water-soluble components (<30, <50, and <100 kDa soluble fractions) were incubated separately with insoluble fraction and <10 kDa soluble fraction, ZnPP formation was same as control in all groups (Figure 2b). As the three fractionated molecular weight groups (<30, <50, and <100 kDa soluble fractions) contained the <10 kDa soluble fraction in common, introduction of additional <10 kDa soluble fraction did not lead to formation of additional ZnPP. In other words, soluble <30, <50, and <100 kDa fraction groups contained sufficient amounts of the <10 kDa fractions for optimum ZnPP formation at pH 5.5.
3.4 Effect of exogenous myoglobin on ZnPP formation
The soluble 10–30 kDa fraction that was essential for ZnPP formation in pork at pH 5.5 contains myoglobin (17 kDa). Grossi et al. (2014) reported that the degradation of myoglobin was essential for the formation of ZnPP, but there has not yet been any direct proof of the contribution of myoglobin to ZnPP formation. Therefore, the involvement of myoglobin in ZnPP formation was studied using commercially available myoglobin reagent. In addition, Ishikawa et al. (2006) reported that ZnPP formation was markedly increased by using oxymyoglobin reduced with ascorbate while the increase with metmyoglobin (MMb) was small. Moreover, the formation of higher concentrations of MMb led to less red color in Parma ham (Adamsen, Møller, Laursen, et al., 2006). Therefore, in this study, the reduced state of myoglobin, i.e., oxymyoglobin, was used.
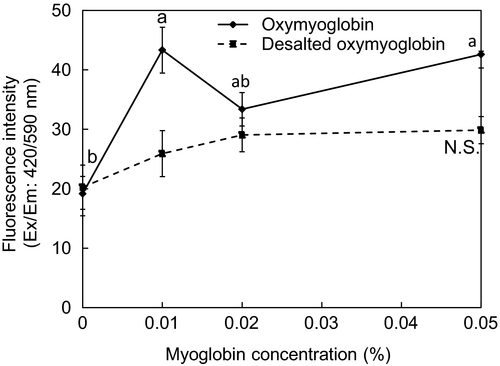
We investigated the supplemental effect of exogenous oxymyoglobin instead of the >10 kDa soluble fraction on ZnPP formation at pH 5.5 (Figure 3). When oxymyoglobin was added at 0.01%, the amount of ZnPP was increased. But, when added in higher concentrations of 0.02% and 0.05%, ZnPP formation was decreased. This result was inconsistent with the result reported by Ishikawa et al. (2006). The myoglobin reagent used in this study might contain impurities (data not shown) and sodium ascorbate is included in oxymyoglobin solution as a reductant. In order to remove these putative impurities, the oxymyoglobin solution was dialyzed overnight through a dialysis tube. When desalted oxymyoglobin was added in the same concentration to the <10 kDa soluble fraction and insoluble fraction, the ZnPP formation amount was not significantly increased. Ishikawa et al. (2007) suggested that mitochondria have the ability to form PPIX from oxymyoglobin and therefore, are directly related to the release of Fe2+ from the porphyrin ring in myoglobin. On the other hand, Wakamatsu et al. (2007) reported that 0.1% of exogenous myoglobin increased ZnPP formation but further addition gradually decreased ZnPP formation. Accordingly, we hypothesize that the impurities in the myoglobin reagent promoted ZnPP formation, but myoglobin itself might not contribute to ZnPP formation at pH 5.5.
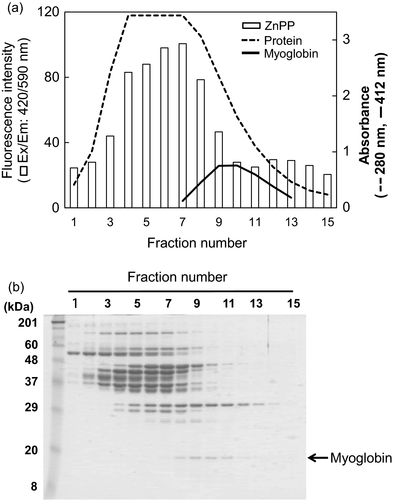
3.5 ZnPP-forming ability of the water-soluble protein fractions separated by gel filtration chromatography
To investigate the contribution of endogenous myoglobin or other water-soluble proteins in the soluble >10 kDa fraction to ZnPP formation, we separated the proteins from the water-soluble fraction using gel filtration chromatography and applied them to the ZnPP formation model experiment system. The ZnPP-forming ability of the separated water-soluble fractions and SDS-PAGE of the eluted fractions are presented in Figure 4a,b, respectively. The results show that the high ZnPP-forming fractions and myoglobin-containing fractions are different (Figure 4a). ZnPP was formed in earlier eluted fractions compared with myoglobin-containing fractions. This suggests that the molecular weight of the contributors to ZnPP formation is higher than that of myoglobin. Khozroughi et al. (2017) reported that ZnPP is formed by a Fe2+-Zn2+ substitution in myoglobin-heme where accompanying myoglobin degradation is not necessarily obligatory and other proteins might be involved in the formation of ZnPP. However, in our earlier study, we found that ZnPP in a model solution at pH 5.5 was not formed by Fe-Zn substitution in heme but was formed by the insertion of Zn into PPIX which was formed independently (Wakamatsu et al., 2007). The data in this study would support our previous study. Therefore, the present result suggests that proteins with molecular weights higher than that of myoglobin in the soluble 10–30 kDa fraction contribute to ZnPP formation. Recently, another mechanism with a different optimum pH (4.75) for the formation of ZnPP in pork was discovered by Wakamatsu et al. (2019). Contributors to ZnPP formation in pork at pH 4.75 might be different from those at pH 5.5.
4 CONCLUSIONS
The present study revealed that three or more components present across the >10 kDa soluble fraction, the <10 kDa soluble fraction, and the insoluble fraction of pork are essential to ZnPP formation at pH 5.5. In addition, heat-resistant components in the <10 kDa soluble fraction contribute to ZnPP formation. Exogenous myoglobin and separated endogenous myoglobin by gel filtration did not promote the formation of ZnPP in pork homogenate at pH 5.5. Our study suggests that sarcoplasmic proteins with molecular weights greater than that of myoglobin contribute to ZnPP formation. Although the present research did not ascertain the identity of the water-soluble protein and low molecular weight components contributing to ZnPP formation, it will, nevertheless, be a milestone in the elucidation of the ZnPP formation mechanism at pH 5.5 as well as in the identification of the water-soluble protein(s) contributing to this process.
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI Grant Number JP20580288. We acknowledge Editage (www.editage.jp) for English language editing.




