β-carotene attenuates lipopolysaccharide-induced inflammation via inhibition of the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages
Abstract
β-carotene is one of the most abundant carotenoids, has potential anti-inflammatory effect, it has been reported that β-carotene could suppress LPS-induced inflammatory responses by inhibiting nuclear factor kappa B (NF-κB) translocation, but the more detailed molecular mechanisms underlying the anti-inflammatory action of β-carotene remain to be fully understood. In this study, we investigated the influence of β-carotene on the activation of JAK2/STAT3, MAPK, and NF-κB signaling pathway induced by LPS in RAW264.7 cells and peritoneal macrophages. Cells were treated with different concentrations of β-carotene for 3 hr after LPS treatment for 24 hr. The mRNA expression and the release of IL-1β, IL-6, and TNF-α were evaluated by RT-PCR and ELISA, and the level of signaling proteins of JAK2/STAT3, MAPK, and NF-κB signaling pathway were detected by Western blot. The results showed that β-carotene significantly suppressed (p < 0.05) LPS-induced release of IL-1β, IL-6, and TNF-α and their mRNA expression. LPS-induced JAK2/STAT3, IκB/NF-κB p65, JNK/p38 MAPK signal activation were significantly attenuated (p < 0.05) by β-carotene in a dose-dependent manner. In conclusion, β-carotene could attenuate LPS-induced inflammation via inhibition of the NF-κB, JAK2/STAT3, and JNK/p38 MAPK signaling pathways in macrophages.
1 INTRODUCTION
Inflammation is a defence mechanism activated following certain environmental stimuli, including infection, toxin exposure, or tissue injury. Lipopolysaccharide (LPS) is an endotoxin produced by gram-negative bacteria. Located in the cell wall, it can stimulate various host cells to respond to inflammation. LPS activates macrophages and causes them to secrete a variety of pro-inflammatory cytokines, including TNF-α, IL-6, IL-1β, NO, and PGE2 (Qi et al., 2013). The accumulation of pro-inflammatory mediators is involved in many human diseases associated with inflammation, such as asthma, atherosclerosis, and arthritis (Hayashida, Parks, & Park, 2009; McCartney-Francis et al., 1993).
As primary phagocytic cells, macrophages play a key role in host defence and regulating inflammation (Shin et al., 2011); macrophages are activated by pro-inflammatory factors that in turn activate several intracellular signaling pathways, including the Janus kinase 2/signal transducers and activators of transcription 3 (JAK2/STAT3), mammalian mitogen-activated protein kinase (MAPK), and nuclear factor-kappaB (NF-κB) pathways (Cho, Lim, & Kim, 2013; Guo et al., 2014; Verstrepen et al., 2008). JAK2 and its downstream factor STAT3 (JAK2/STAT3 signaling pathway) are triggered by ligand (i.e., LPS) stimulation, at which time activated STAT3 translocates into the nucleus to regulate the expression of target genes (Kou, Qi, Dai, Luo, & Yin, 2011). MAPKs are a family of serine/threonine protein kinases that play a critical role in innate immune signaling (Dong, Davis, & Flavell, 2002; Tang et al., 2011). Activated NF-κB can translocate into the nucleus to induce the expression of pro-inflammatory genes (Gilroy, Lawrence, Perretti, & Rossi, 2004) and has been implicated in several inflammatory diseases (Makarov, 2000). Thus, suppressing the release of such pro-inflammatory cytokines is essential for the treatment or regulation of inflammatory disease.
β-carotene, an isoprenoid, is widely distributed in green leafy vegetables and yellow- and orange-colored vegetables, as well as in orange-colored fruits. It is one of the most abundant carotenoids (Kameji, Mochizuki, Miyoshi, & Goda, 2010; Lakshminarayana, Raju, Krishnakantha, & Baskaran, 2005; Priyadarshani & Jansz, 2014). As a precursor of vitamin A, β-carotene exhibits antioxidant, anti-inflammatory, and anti-cancer activities (Guo et al., 2015; Niranjana et al., 2015), and has been shown to play a role in the prevention of cardiovascular disease (Gaziano et al., 1995). β-carotene has been reported to suppress LPS-induced inflammatory responses by inhibiting nuclear factor kappa B (NF-κB) translocation in RAW264.7 cell lines (Bai et al., 2005). In addition, β-carotene enhances mucosal IgA induction in weanling mice (Nishida, Sugimoto, Ikeda, & Kume, 2014). Nevertheless, the detailed molecular mechanisms underlying the anti-inflammatory activity of β-carotene remain to be elucidated. In addition to NF-κB, STAT and MAPK are important transcription factors involved in immunity and inflammatory pathways (Galdiero, Vitiello, D'isanto, Raieta, & Galdiero, 2006; Kyriakis & Avruch, 2001). Thus, our goal was to explore the inhibitory effects and the underlying mechanisms of β-carotene on the expression of inflammatory mediators upon LPS stimulation in RAW264.7 cells and peritoneal macrophages.
In the present study, we investigate the influence of β-carotene on the activation of the JAK2/STAT3, MAPK, and NF-κB signaling pathways following LPS induction in RAW264.7 cells and peritoneal macrophages. Our results showed that β-carotene attenuates LPS-induced the release of pro-inflammatory cytokines and mediators through the suppression of the JAK2-STAT3, NF-κB, and JNK/p38 MAPK signal pathways. To our knowledge, this is the first study of the role of β-carotene in the suppression of LPS-induced JAK2/STAT3 and MAPK activity. Our study provides new insight into the anti-inflammatory mechanism of β-carotene.
2 MATERIALS AND METHODS
2.1 Antibodies and reagents
β-carotene and LPS were purchased from Sigma-Aldrich Co. (USA). Rabbit monoclonal antibodies for phospho-JAK2 and JAK2; phospho-STAT3 and STAT3; phospho-JNK and JNK; phospho-ERK1/2 and ERK1/2; NF-κB p65; IκBα; phosphor-IκBα; β-actin; lamina; HRP-conjugated anti-rabbit IgG; HRP-conjugated anti-mouse IgG; and FITC-conjugated anti-rabbit IgG were obtained from Cell Signaling Technology (Danvers, MA, USA). Cell lysis buffer (RIPA kit), Difco skim milk, enhanced chemiluminescence (ECL) and BCA kits were obtained from Pierce (USA). RPMI 1640 and fetal bovine serum (FBS) were obtained from Gibco (USA). Bovine serum albumin (BSA) and polyvinylidene fluoride (PVDF) membranes were obtained from Millipore (Bedford, MA, USA). Goat anti-Rabbit IgG H&L (FITC) was purchased from Abcam (UK). Unless otherwise stated, other reagents were obtained from Sigma (USA).
2.2 Cell line, murine peritoneal microphage isolation, and cell culture
The murine macrophage cell line RAW264.7 was maintained in RPMI 1640 supplemented with 10% FBS, streptomycin (0.05 mg/ml), and penicillin (0.1 mg/ml) (Biofluids, Rockville, MD, USA) at 37°C in a 100% humidified atmosphere of 5% CO2.
Murine peritoneal microphages were prepared as previously described with slight modifications (Guan et al., 2012). The isolation method was approved by the Animal Ethical Committee of Jilin Agricultural University. Briefly, BALB/c mice (6- to 8-week-old females weighing 20 ± 2 g, provided by the Experimental Animal Center, Jilin University) were anesthetized using 4% chloral hydrate (0.1 ml/10 g). Five ml RPMI 1640 supplemented with streptomycin (0.05 mg/ml) and penicillin (0.1 mg/ml) was intraperitoneally injected, and peritoneal lavage fluid was extracted using a sterile pipette after the abdomen was gently kneaded for 2–3 min. The peritoneal lavage fluid was centrifuged twice at 1,000 RPM for 5 min, and the precipitate was resuspended in RPMI 1,640 supplemented with 10% FBS, streptomycin (0.05 mg/ml), and penicillin (0.1 mg/ml). The media were changed after 4 hr, when the floating murine peritoneal microphages had attached to collagen-coated plastic culture dishes.
2.3 Cell viability assays
Cell viability was determined by MTT assay. The cells were plated in 96-well plates at a density of 5,000 cells/well to evaluate the LPS dose–response and the effect of β-carotene on LPS-induced cytotoxicity. RAW264.7 cells and peritoneal macrophages were treated with LPS (100 ng/ml) for 24 hr then were incubated with β-carotene (10, 25, 50, 75, and 100 μM) for 3 hr. Untreated RAW264.7 cells and peritoneal macrophages were used as a control. MTT solution (150 μl, 0.5 mg/ml) was added to each well, and the cells were incubated at 37°C for 4 hr. The medium was removed, and 100 μl of DMSO was added to each well for 15 min. The product was measured using a microplate reader (Thermo Scientific, Multiskan FC, Pittsburgh PK).
2.4 Pro-inflammatory cytokines secretion and mRNA expression
RAW264.7 cells and peritoneal macrophages were treated with LPS (100 ng/ml) for 24 hr, and then incubated with β-carotene (50, 75 and 100 μM) for 3 hr. Untreated cells were used as a control. To evaluate cytokine concentrations (IL-6, IL-1β, and TNF-α), cell culture medium supernatants were collected and probed using Enzyme-linked immunosorbent assay (ELISA) kits (R&D systems) according to the manufacturer's instructions.
- IL-6: F: TGGAAATGAGAAAAGAGTTGTGC, R: CCAGTTTGGTAGCATCCATCA;
- IL-1β: F: TTCATCTTTGAAGAAGAGCCCAT, R: TCGGAGCCTGTAGTGCAGTT;
- TNF-α: F: GTGATCGGTCCCCAAAGG, R: GGTGGTTTGCTACGACGTG;
- β-actin: F: CGGGGACCTGACTGACTACC, R: AGGAAGGCTGGAAGAGTGC.
2.5 Measurement of inflammation-related proteins expression in cells
Nuclear and cytosolic protein of RAW264.7 cells and peritoneal macrophages were isolated using nuclear and cytosol extraction kits according to the manufacturer's instructions. Isolated proteins were subjected to Western blot analyses as described below.
Western blotting was performed as previously described (Hong, Lan, Li, Fu, & Zheng, 2016). Briefly, protein samples were fractionated using SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked for 1 hr at 37°C with 5% bovine serum albumin and were incubated overnight at 4°C with primary antibodies according to the manufacturer's protocols. The membranes were incubated with secondary antibodies conjugated with horseradish peroxidase at room temperature for 1 hr after washing three times. After a final three washes, the immunoreactive protein bands were detected using an ECL detection system.
2.6 Densitometric analysis
Densitometric analysis of the immunoreactive protein bands was performed using Quantity One® software (developed by BioRad Technical Service Department, USA; [email protected]).
2.7 Statistical analysis
All data are represented as mean values ± SE. Statistical analysis was performed using ANOVA and Tukey’ s multiple comparisons test with Statistical Analysis System (SAS) software (SAS version 9.0, Institute Inc., Cary, NC, USA).
3 RESULTS
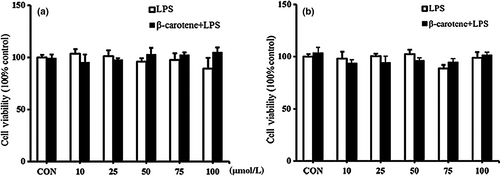
3.1 Effects of β-carotene on the viability of RAW264.7 cells and peritoneal macrophages
In our work, we found that β-carotene did not significantly affect the viability of RAW264.7 cells and peritoneal macrophages (Figure 1a, b). Thus, the effects of β-carotene on RAW264.7 cells and peritoneal macrophages are not attributable to cytotoxicity. We used between 50 and 100 μM of β-carotene for subsequent experiments.
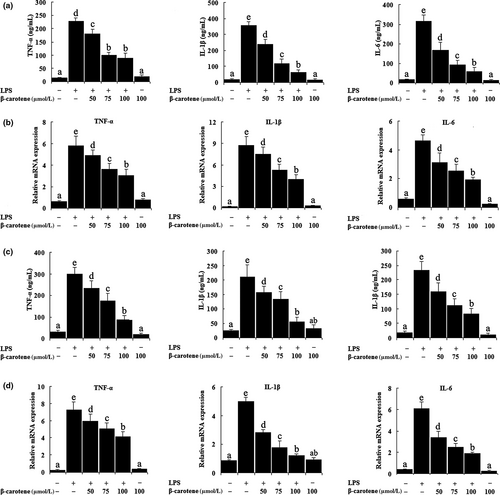
3.2 β-carotene decreases the production of pro-inflammatory cytokines induced by LPS
As shown in Figure 2a, c, LPS significantly increased (p < 0.05) the secretion of IL-1β, IL-6, and TNF-α in the culture medium, whereas β-carotene could inhibit the release of these cytokines in a dose-dependent manner, and the best effect on inhibitory is the concentration of 100 μM. In addition, the results showed that β-carotene significantly reduced (p < 0.05) the expression of IL-1β, IL-6, and TNF-α mRNA following LPS induction in RAW264.7 cells (Figure 2b). We observed the same effect in LPS-treated peritoneal macrophages. The levels of IL-1β, IL-6, and TNF-α mRNA were markedly augmented (p < 0.05) by LPS treatment, whereas they were reduced (p < 0.05) by β-carotene significantly (Figure 2d).
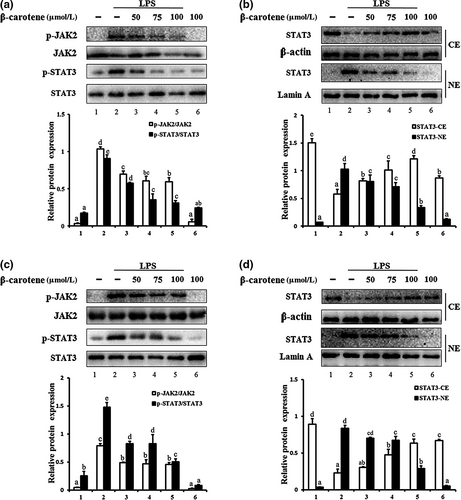
3.3 β-carotene suppressed LPS-induced JAK2/STAT3 pathway activity
As shown in Figure 3a, LPS treatment markedly increased (p < 0.05) JAK2 and STAT3 phosphorylation in RAW264.7 cells, which was inhibited by β-carotene in a dose-dependent manner. We observed that β-carotene has inhibitory effect in concentration of 50 μM and the optimal inhibitory concentration is 100 μM. Once activated, STAT3 translocates to the nucleus. We therefore examined whether β-carotene inhibits the nuclear translocation of STAT3. Nuclear and cytoplasmic proteins were extracted and detected. The level of STAT3 protein following LPS induction was clearly increased (p < 0.05) in the nuclear extract, whereas the nuclear translocation of STAT3 was attenuated (p < 0.05) by β-carotene markedly (Figure 3b). The same phenomenon was also observed for LPS-induced peritoneal macrophages (Figure 3c and D). Thus, β-carotene could suppress LPS-induced JAK2-STAT3 signal activation in RAW264.7 cells and peritoneal macrophages.
3.4 3.4 β-carotene suppressed LPS-induced NF-κB pathway and MAPK pathway activity
We investigated the effect of β-carotene on the NF-κB pathway. We found that the IκB/NF-κB p65 signaling pathway was significantly activated (p < 0.05) by LPS in RAW264.7 cells and peritoneal macrophages. Moreover, IκBα phosphorylation and the translocation of p65 were significantly attenuated (p < 0.05) by β-carotene in a dose-dependent manner (Figure 4a-d). β-carotene (50 μM) has effect on suppressing IκBα phosphorylation and the nuclear translocation of p65, β-carotene (75 μM) on a middle suppression level probably, and the best effect on inhibitory is the concentration of 100 μM.
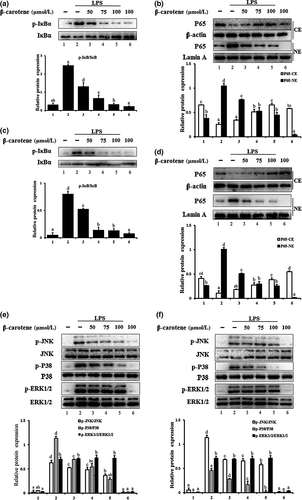
We also examined the effect of β-carotene on the LPS-induced phosphorylation of JNK, ERK1/2, and p38 in RAW264.7 cells and peritoneal macrophages. Both JNK and p38 were significantly inhibited (p < 0.05) by β-carotene in a dose-dependent manner in LPS-induced RAW264.7 cells and peritoneal macrophages. However, treatment with β-carotene failed to influence the LPS-activated phosphorylation of ERK1/2 (Figure 4e, f).
4 DISCUSSION
Inflammation is the body's adaptive response to pathogens, injuries, or other stressors. The body must strive to achieve equilibrium during the inflammatory response, or the excessive or unregulated expression of pro-inflammatory cytokines will result in tissue injury (A Ivanenkov, Balakin, & Lavrovsky, 2011). A powerful antioxidant, anti-inflammatory, and anti-cancer compound (Guo et al., 2015; Niranjana et al., 2015), β-carotene has been reported to prevent cardiovascular disease (Gaziano et al., 1995) and suppress lipid peroxidation (Everett et al., 1996). β-carotene exerts an anti-inflammatory effect by inhibiting NF-κB nuclear translocation (Bai et al., 2005). Nevertheless, the detailed molecular mechanism underlying the anti-inflammatory action of β-carotene remains unclear.
In the present study, we investigated the anti-inflammatory properties of β-carotene in LPS-induced RAW264.7 cells and peritoneal macrophages. As immune cells, macrophages play a key role in the occurrence and development of inflammation (Hoffmann, Kafatos, Janeway, & Ezekowitz, 1999). Thus, they are often used as a model to study the anti-inflammatory mechanisms of anti-inflammatory compounds. IL-6 acts as a pro-inflammatory cytokine by activating the JAK2/STAT3 signaling pathway. Katsuura et al. found that β-carotene suppressed the transcription of IL-1β and IL-6 cytokines in RAW264 cells (Katsuura, Imamura, Bando, & Yamanishi, 2009). We found that β-carotene suppresses the production of several pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, induced by LPS treatment, which is consistent with Katsuura's report.
Toll-like receptor (TLR) 4 interacts with LPS and initiates an inflammatory signaling cascade. NF-κB is inhibited by inhibitory factor IκB at rest. Once activated (i.e., following LPS treatment), the NF-κB p65 subunit is released after IκB phosphorylation. Phosphorylated NF-κB p65 translocates to the nucleus to regulate target gene transcription, including numerous inflammatory mediators and cytokines (e.g., TNF-α, IL-6, IL-1β, NO, and PGE2) (Hoffmann & Baltimore, 2006; Weng et al., 2017). Milani et al. reported that β-carotene blocks nuclear translocation of the NF-ĸB p65 subunit which is correlated with its inhibitory effect on phosphorylation and degradation of the NF-ĸB inhibitor (Milani, Basirnejad, Shahbazi, & Bolhassani, 2017). As shown in Figure 4a-d, β-carotene attenuates the level of IκB phosphorylation and inhibits NF-κB p65 nuclear translocation significantly. These results suggest that the anti-inflammatory effect of β-carotene is achieved by suppressing the nuclear translocation of NF-κB p65 through blocking IκB phosphorylation and degradation, which is consistent with Bai's report (Bai et al., 2005). Until now, the exact molecular target site of β-carotene is not clear and it may have multiple target sites. Therefore, we cannot rule out the possibility that the inhibition of NF-kB pathway by β-carotene is through inhibiting the DNA binding activity of NF-kB p65. Besides, it may prevent the interaction between the p65 subunit of NF-kB and the transcriptional co-activator CBP to inhibit the activity of NF-kB transcriptional.
JAK2/STAT3 signaling pathway is involved in the inflammatory response (Cho et al., 2013), and small molecular inhibitors of the JAK2/STAT3 signaling pathway have attracted much attention in the context of treating inflammatory diseases (Qi, Qi, Ling, Lv, & Feng, 2016; Seo, Jeong, Lee, Jang, & Choi, 2016; Weng et al., 2017). In our study, LPS treatment increased JAK2, STAT3 phosphorylation in RAW264.7 cells and peritoneal macrophages. However, we evaluated the effects of β-carotene on JAK2/STAT3 activation and found that β-carotene could attenuate the phosphorylation of JAK2 and STAT3, it suggested that β-carotene might exert its anti-inflammatory effects through inhibition of the JAK2/STAT3 signaling pathway. Speranza et al. showed that astaxanthin, a carotenoid found in marine animals and vegetables, reduces activation of NF-ĸB induced by H2O2 and secretion of its cytokines through modulation of SHP-1 expression. SHP-1, acts as a negative regulator, is a tyrosine phosphatase (PTP) and it could negative regulation of cytokine signaling pathway (Speranza et al., 2012). We cannot exclude that the negative regulators of JAK/STAT pathway were activated by β-carotene, such as suppressors of cytokine signaling (SOCS) family and PTPs, which inhibited JAK/STAT pathway.
The MAPK family of serine/threonine protein kinases consists of the p42/p44 ERKs, JNKs, and p38 MAPKs. The signaling pathways downstream of MAPKs are involved in cell proliferation, inflammation, and apoptosis. It has been reported that LPS induces the phosphorylation of ERK1/2, JNK, and p38 MAPKs in murine macrophages (Bode, Ehlting, & Häussinger, 2012; Ruimi et al., 2010). In our study, LPS activated the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways, β-carotene attenuated the level of NF-κB, JAK2, STAT3, JNK, and p38 phosphorylation. However, β-carotene had no effect on phosphor-ERK1/2. Shree et al. reported that the phosphorylation of ERK1/2 intracellular proteins has been restrained by β-carotene in human breast cancer (MCF-7) cells (Shree et al., 2017). Kavitha et al. showed that astaxanthin reduced NF-κB signaling by inhibiting the activity of ERK/MAPK and phosphatidylinositol-3-kinase (PI-3K) /Akt in the hamster buccal pouch (HBP) carcinogenesis model (Kavitha, Kowshik, Kishore, Baba, & Nagini, 2013). We testified that the anti-inflammatory effect of β-carotene might be achieved by attenuating the phosphorylation level of JNK and p38, but had no effect on phosphor-ERK1/2. It was not in accordance with Shree et al. and Kavitha et al. studies, which found that few carotenoids reduced the ERK1/2 activation. We speculate that it may be due to the cell models we used differently.
Moreover, Yu et al. testified that resokaempferol attenuates the activation of JNK and p38 but not ERK in LPS-induced inflammation RAW264.7 cells to achieve the anti-inflammatory effect (Yu et al., 2016). Lin et al. also did not observe a significant alteration of ERK1/2 phosphorylation in human neuroblastoma SH-SY5Y cells treated by either glutamate or astaxanthin (Lin, Zhao, & Li, 2017). These studies might support the information for no change in ERK1/2 activation upon some natural compounds treatment. The anti-inflammatory effect of β-carotene might be achieved by inhibiting the activation of JNK and p38 but not ERK1/2 in LPS-induced RAW264.7 cells and peritoneal macrophages under our experimental conditions. We speculated that it may be because the ERK1/2 signaling cascade is activated by a wide variety of receptors involved in growth and differentiation, including receptor tyrosine kinases (RTKs), integrins, and ion channels (Keshet & Seger, 2010; Wainstein & Seger, 2016).
In summary, our results showed that β-carotene attenuates the LPS-induced inflammation in RAW264.7 cells and peritoneal macrophages, particularly by inhibiting multiple signaling pathways, such as JAK2/STAT3, NF-κB and JNK/p38 MAPK but not ERK1/2. This study enriches our knowledge about β-carotene's anti-inflammatory mechanism and highlights a therapeutic potential of β-carotene in treating inflammatory diseases. However, the reason that the anti-inflammatory mechanism of β-carotene is not fully understood and further studies are necessary, which is also the direction of our next work.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant Nos.31672511).




