Advanced treatment technique for swine wastewater using two agents: Thermally polymerized amorphous silica and hydrated lime for color and phosphorus removal and sulfur for nitrogen removal
Abstract
The efficacy of advanced treatment of swine wastewater using thermally polymerized, modified amorphous silica and hydrated lime (M-CSH-lime) for color and phosphorus removal and sulfur for nitrogen removal was examined with a demonstration-scale treatment plant. The color removal rate was approximately 78% at M-CSH-lime addition rates of > 0.055 wt/v%. The 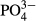 -P removal rate exceeded 99.9% with > 0.023 wt/v%. pH of the effluent from the M-CSH-lime reactor increased with the addition rate till a maximum value of 12.7, which was effective in disinfection. The recovered M-CSH-lime would be suitable as a phosphorus fertilizer because the total P2O5 content was approximately 10%. The nitrogen oxide (NOx-N) removal rate by sulfur denitrification increased to approximately 80% when the NOx-N loading rate was around 0.1 kg-N/ton-S/day. It was suggested that the combination of the two processes would be effective in the advanced treatment of swine wastewater.
-P removal rate exceeded 99.9% with > 0.023 wt/v%. pH of the effluent from the M-CSH-lime reactor increased with the addition rate till a maximum value of 12.7, which was effective in disinfection. The recovered M-CSH-lime would be suitable as a phosphorus fertilizer because the total P2O5 content was approximately 10%. The nitrogen oxide (NOx-N) removal rate by sulfur denitrification increased to approximately 80% when the NOx-N loading rate was around 0.1 kg-N/ton-S/day. It was suggested that the combination of the two processes would be effective in the advanced treatment of swine wastewater.
Introduction
In Japan, wastewater from swine farms is mostly treated with an activated sludge process. Sufficient removal of color, phosphorus and nitrogen from swine wastewater simultaneously with an acceptable cost is an important topic that needs to be considered in the livestock industry in Japan. In general, the color of an effluent from a swine wastewater treatment facility is dark brown, even after sufficient biological treatment. This color frequently leads to the misunderstanding that the effluent is untreated. Activated carbon adsorption (Choy et al. 1999) and ozonation (Namboodri et al.1994) are the most typical methods used for color removal. However, the use of these decolorization methods is difficult in the livestock industry because of their high initial and running costs.
The discharge of effluent containing a high concentration of phosphorus causes the eutrophication of lakes and marshes. On the other hand, the recovery of phosphorus, which is an exhaustible resource, is necessary (Gilbert 2009). This is particularly important in Japan, where most phosphate rocks are imported. The process of recovery of phosphorus by the struvite crystallization process has been developed in the livestock industry (Suzuki et al. 2006; Kawamura et al. 2011). However, the phosphorus removal rate by this process is not high enough to prevent eutrophication. For example, the effluent standard for phosphorus in the watershed of Lake Kasumigaura in Ibaraki Prefecture, Japan, is 1–6 mg/L. Maintaining this effluent standard using the struvite crystallization process is difficult.
In general, the effluent from a swine wastewater treatment facility also contains a high concentration of nitrogen oxide (NOx-N), which would increase groundwater pollution (Unisuga & Endo 2011) and the hazards associated with drinking water (Freitas et al. 2001). Biological denitrification processes such as intermittent aeration have been widely applied to the swine wastewater treatment process (Osada et al. 1991). However, Waki et al. (2010) reported that biochemical oxygen demand (BOD)/N ratios of wastewater in many swine wastewater treatment facilities are lower than the value sufficient for denitrification. The denitrification method using anammox bacteria has been actively studied in recent years (Strous et al. 1998). Anammox bacteria have no BOD requirement for denitrification because they use NH4+ as an electron donor for denitrification. However, in livestock wastewater treatment, controlling the appropriate NO2−-N/NH4+-N ratio, which is a prerequisite for the anammox process, is difficult.
To cope with these challenges, it is crucial to develop new treatment processes with a simple structure and low initial and operating costs. Yamashita et al. (2013) reported that an agent synthesized from amorphous silica and hydrated lime (CSH-lime) showed sufficient efficacy with regard to color and phosphorus removal and disinfection at an addition rate of over 0.5 wt/v%. CSH-lime is a highly alkaline material and was synthesized using amorphous silica derived from diatomaceous earth and hydrated lime. We modified CSH-lime (M-CSH-lime) and succeeded in obtaining sufficient efficacy at a lower addition rate than CSH-lime (Hasegawa et al. 2014a). A bench-scale test with M-CSH-lime exhibited its effectiveness in simultaneous color and phosphorus removal and disinfection at an addition rate of 0.15 wt/v%. Recovered M-CSH-lime after treatment contained over 15% phosphorus (Tanaka et al. 2014; Hasegawa et al. 2014a), a value comparable to that of phosphorus fertilizers.
In the present study, we installed a demonstration-scale treatment plant using M-CSH-lime in a swine farm to examine the practical efficacy and cost. The plant also included an autotrophic denitrification process using sulfur (autotrophic sulfur denitrification (ADS) reactor) after the M-CSH-lime treatment process. Sulfur denitrification is a biological process promoted by sulfur-denitrifying bacteria, which oxidizes sulfur with NO3− under anoxic conditions, resulting in the production of 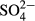 and N2. Hasegawa and Tanaka (2015a) reported that a baffled reactor filled with sulfur powder removed over 78% of NOx-N at an NOx-N loading rate of 0.4 kg-N/ton-S/day. The denitrification process with sulfur requires a carbonate ion for the growth of sulfur-denitrifying bacteria and an alkaline agent for the neutralization of formed sulfate ions. Calcium carbonate or sodium bicarbonate is generally used for these purposes. M-CSH-lime contains free hydrate lime, which will change in liquid to calcium carbonate. Therefore, M-CSH-lime may also promote the sulfur denitrification process. This suggests an advantage of the combination of M-CSH-lime and ADS processes. We present the results of the operation of a plant consisting of an M-CSH-lime reactor and a subsequent ADS reactor.
and N2. Hasegawa and Tanaka (2015a) reported that a baffled reactor filled with sulfur powder removed over 78% of NOx-N at an NOx-N loading rate of 0.4 kg-N/ton-S/day. The denitrification process with sulfur requires a carbonate ion for the growth of sulfur-denitrifying bacteria and an alkaline agent for the neutralization of formed sulfate ions. Calcium carbonate or sodium bicarbonate is generally used for these purposes. M-CSH-lime contains free hydrate lime, which will change in liquid to calcium carbonate. Therefore, M-CSH-lime may also promote the sulfur denitrification process. This suggests an advantage of the combination of M-CSH-lime and ADS processes. We present the results of the operation of a plant consisting of an M-CSH-lime reactor and a subsequent ADS reactor.
Materials and methods
Time and place of examination
The plant was installed in a swine wastewater treatment facility in Chiba Prefecture, Japan. Biologically treated wastewater, which was discharged from the membrane bioreactor process of this facility, was used as the plant influent.
The experiment was conducted for 101 days from 1 September, 2014 to 10 December, 2014.
Treatment agent
M-CSH-lime: diatomaceous earth was added to NaOH solution, and the dissolved silica solution was obtained by heating this at 70°C for 1 h. Hydrated lime was added to the dissolved silica, which adjusted pH to 10.3, and it was mixed using a magnetic stirrer at 70°C for 1 h (Hasegawa et al. 2014a). The calcium content in M-CSH-lime was 60%.
Sulfur: commercially available sulfur powder (99% purity, 200 mesh pass; Berry's Life Co., Kagawa, Japan), which is usually applied, for example, as a soil pH adjustor, was used for the experiment. Because the sulfur powder was hydrophobic, household dishwashing detergent liquid was added to the sulfur slurry (10 mL/kg sulfur) and mixed well. As reported by Tanaka et al. (2013), sulfur powder becomes hydrophilic after this procedure and precipitates at the bottom of the reactor.
Flow diagram of the plant
The plant consisted of an M-CSH-lime reactor and an ADS reactor (Fig. 1). The M-CSH-lime reactor was made of polyvinyl chloride. It was cylindrical in shape, with a diameter of 63 cm, height of 3 m and effective volume of 0.832 m3. The storage tank of M-CSH-lime slurry was 1 m3 with continuous aeration to prevent sedimentation. The liquid overflowed from the upper nozzle of the reactor. To prevent a short passage of liquid through the bed layer of the accumulated M-CSH-lime, aeration was applied for a minute every day to homogenize the bed layer.
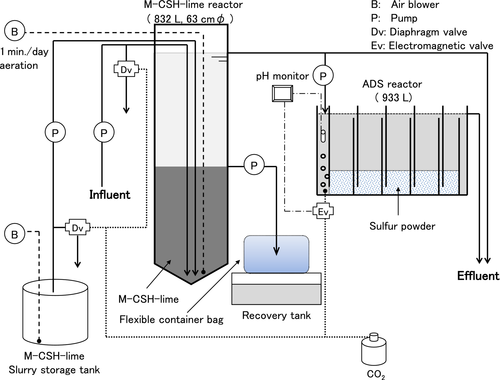
A part of the overflown liquid was fed to the ADS reactor after neutralization with CO2 gas. The ADS reactor consisted of three baffled tanks connected in a series. Each tank (effective volume of 0.311 m3) consisted of seven chambers separated with a series of vertical baffles that directed the wastewater under and over the baffles as it passed from the inlet to the outlet. Thus, the structure enabled the wastewater to come into intimate contact with the sulfur powder as it passed through the chambers of the water tank. In total, 40 kg of sulfur powder (nominal volume of 56 L) was filled in the reactor.
The CO2 gas injection ratio was 120–320 mL/min per 30–633 mL/min of liquid. The liquid then came in contact with the sulfur powder as it passed through the chambers of the reactor and overflowed as the effluent.
The plant was equipped with several mechanisms for preventing the discharge of alkaline liquid into public water bodies. First, a gas pressure diaphragm valve was installed in the M-CSH-lime slurry injection line and the influent feed line and was operated with a pressure CO2 gas cylinder and control. When the cylinder was empty, the valves automatically opened and the feed of M-CSH-lime and influent came to a halt, thereby preventing the discharge of non-neutralized water from the plant. An electromagnetic valve controlled by an effluent pH monitor was then installed in the CO2 gas injection lines to optimize the gas feeding rate.
Operation of the plant
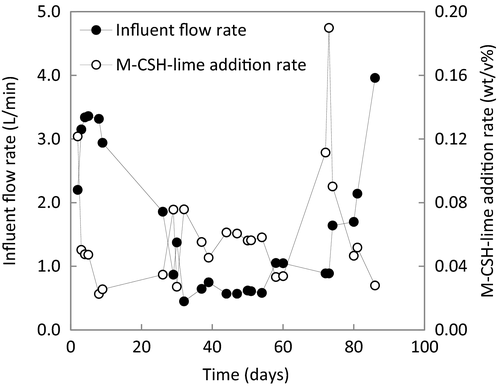
M-CSH-lime reactor: the influent and M-CSH-lime slurry (3.8–7.7 wt/v%) were continuously fed into the M-CSH-lime reactor with tube pumps. The flow rates of the influent and M-CSH-lime slurry were 0.6-5.7 m3/day and 10.8–86.4 L/day, respectively. The nominal hydraulic retention time (HRT) fluctuated from 3.5 to 30.4 h. The M-CSH-lime addition rate was 0.023–0.19 wt/v% (Fig. 2).
Recovery of the phosphate absorbed by the M-CSH-lime was performed as follows. The M-CSH-lime slurry, which accumulated on the bottom of the M-CSH-lime reactor, was transferred into a flexible container bag (effective volume of 0.65 m3) every 6 h by a pump for a few minutes (9 L/min). After the bag was filled with the slurry, it was left to rest for a week for dewatering. The liquid part of the transferred slurry penetrated the cloth of the bag, while the solid part accumulated inside the bag (recovered M-CSH-lime).
ADS reactor: the influent was fed at 30–633 mL/min. As shown in Table 1, the water temperature was between 6.8°C and 21.3°C. The NOx-N loading rates ranged from 0.05 to 5.78 kg-N/ton-S/day. The nominal contact time for the settled sulfur powder layer (HRT corresponding to the nominal bed volume of the sulfur powder layer) was 1.5–15.1 h. Inoculation of a sulfur oxidizing denitrifier was not performed.
| Ave. ± SD (min.–max.) | |
|---|---|
| Water temperature, °C | 14.6 ± 5.1 (6.8–21.3) |
| NOx-N loading rate, kg-N/ton-S/day | 1.84 ± 1.97 (0.05–5.78) |
| HRT (h) | 6.5 ± 4.1 (1.5–15.1) |
- Ave, average; HRT, hydraulic retention time; SD, standard deviation.
Analytical methods for water quality
pH was determined using a glass electrode. BOD was determined using a BOD Trak (Hach Co., Loveland, CO, USA). Suspended solids (SS) were determined according to Standard Methods for the Examination of Water and Wastewater (Clescerl et al. 1998) using a glass-fiber filter (GS-25; Advantec, Tokyo, Japan). The concentrations of 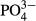 -P, NO3−-N, NO2−-N and
-P, NO3−-N, NO2−-N and 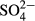 -S were determined by an ion chromatograph (IC-2010; Tosoh, Tokyo, Japan) attached to a TSKgel Super IC-Anion HS column (Tosoh). The concentrations of NH4+-N were determined by an ion chromatograph (DX-120 analyzer; Dionex, Osaka, Japan) attached to an IonPac CS12A column (Dionex). The color was determined by measuring the absorbance at 390 nm (UVmini-1240; Shimadzu, Kyoto, Japan) after filtration through a 0.45 μm filter (IC Millex-LH; Millipore, Billerica, MA, USA). Inorganic carbon (IC) was determined using a TOC/TN analyzer (TOC-V CSN; Shimadzu).
-S were determined by an ion chromatograph (IC-2010; Tosoh, Tokyo, Japan) attached to a TSKgel Super IC-Anion HS column (Tosoh). The concentrations of NH4+-N were determined by an ion chromatograph (DX-120 analyzer; Dionex, Osaka, Japan) attached to an IonPac CS12A column (Dionex). The color was determined by measuring the absorbance at 390 nm (UVmini-1240; Shimadzu, Kyoto, Japan) after filtration through a 0.45 μm filter (IC Millex-LH; Millipore, Billerica, MA, USA). Inorganic carbon (IC) was determined using a TOC/TN analyzer (TOC-V CSN; Shimadzu).
Chemical analyses of recovered M-CSH-lime
Nutrient contents (total (T)-P2O5, T-N, T-K2O and T-MgO) in the recovered M-CSH-lime and separated liquid were determined according to the Japanese Food and Agricultural Materials Inspection Center (2012).
Results
Performance of M-CSH-lime reactor
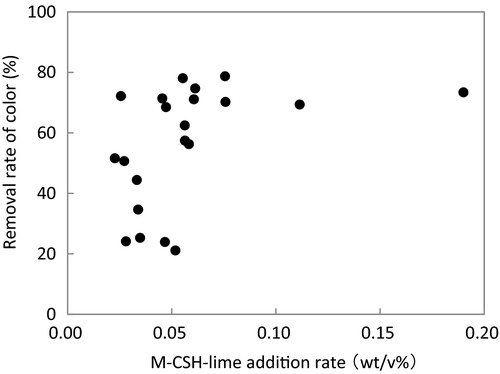
Table 2 shows the water quality of the influent and overflow of the M-CSH-lime reactor during the study period. The average color units decreased from 309.5 in the influent to 142.5 in the overflow (the average removal rate was 55.0%). It has been suggested that 100 units was a sufficiently low value of swine farm effluent from an aesthetic point of view (Watanabe et al. 1997). Therefore, the efficacy with regard to color removal would be satisfactory in the present study. Figure 3 shows the relationship between the M-CSH-lime addition rate and color removal rate. The color removal rate increased with the M-CSH-lime addition rate, reaching a maximum of 78% at an M-CSH-lime addition rate of approximately 0.055 wt/v%.
| Ave. ± SD (min.–max.) | |||
|---|---|---|---|
| Influent of the plant | Overflow of M-CSH-lime reactor | Effluent of ADS reactor | |
| pH | 6.6–8.6 | 7.2–12.7 | 6.9–8.4 |
| pH neutralized with CO2 | – | 7.1–9.4 | – |
| BOD (mg/L) | 5.2 ± 6.1 (0–26.2) | – | 2.5 ± 3.1 (0–8.7) |
| SS (mg/L) | 3.1 ± 5.3 (0.7–27.0) | – | 12.0 ± 7.5 (1.0–36.0) |
| Color (units) | 309.5 ± 36.0 (250.8–370.4) | 142.5 ± 73.6 (60.7–289.1) | – |
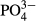 -P (mg/L) -P (mg/L) |
20.9 ± 5.7 (13.6–33.0) | 1.1 ± 2.6 (0–12.4) | – |
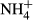 -N (mg/L) -N (mg/L) |
4.8 ± 5.0 (0–15.8) | – | 3.3 ± 3.8 (0.6–14.4) |
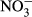 -N (mg/L) -N (mg/L) |
161.1 ± 99.1 (19.8–284.2) | – | 127.3 ± 87.3 (3.7–234.7) |
 -N (mg/L) -N (mg/L) |
1.1 ± 1.0 (0–3.3) | – | 1.9 ± 1.6 (0–4.5) |
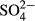 -S (mg/L) -S (mg/L) |
32.8 ± 3.7 (26.9–42.8) | – | 114.0 ± 76.3 (49.3–454.2) |
| IC (mg/L) | 24.5 ± 19.7 (5.6–60.3) | – | 57.7 ± 20.1 (7.9–85.9) |
- Ave, average; ADS, autotrophic sulfur denitrification; BOD, biochemical oxygen demand; IC, inorganic carbon; M-CSH-line, modified amorphous silica and hydrated lime; SD, standard deviation; SS, suspended solids.
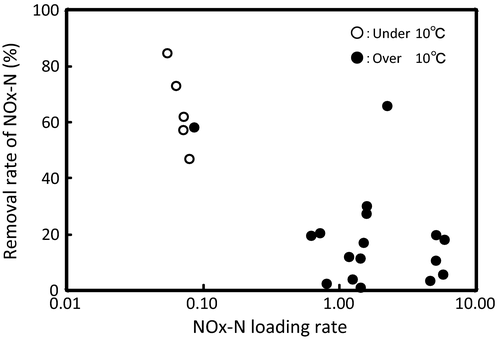
As shown in Table 2, the average 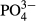 -P concentration decreased from 20.9 mg/L in the influent to 1.1 mg/L in the overflow (the average removal rate was 94.6%). The
-P concentration decreased from 20.9 mg/L in the influent to 1.1 mg/L in the overflow (the average removal rate was 94.6%). The 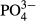 -P removal rate was nearly 100% at an M-CSH-lime addition rate of over 0.023 wt/v% (Fig. 4).
-P removal rate was nearly 100% at an M-CSH-lime addition rate of over 0.023 wt/v% (Fig. 4).
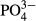 -P removal rate.
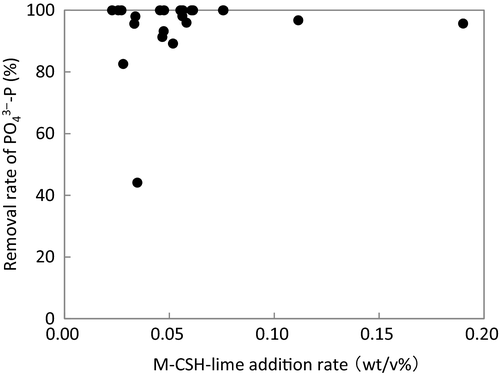
-P removal rate.pH of the influent was 6.6–8.6, and it increased to 7.2–12.7 for the reactor overflow prior to neutralization (Table 2). pH increased with the M-CSH-lime addition rate (Fig. 5). Tanaka et al. (2014) reported that pH of the reactor overflow increased to over 11 with an M-CSH-lime addition rate of 0.1 wt/v%. They also pointed out that this addition rate was effective in decreasing the number of coliform bacteria. In the present study, pH of the reactor overflow increased to approximately 12 at M-CSH-lime addition rates of over 0.05 wt/v%. Therefore, an M-CSH-lime addition rate of 0.05 wt/v% would be effective against coliform bacteria.
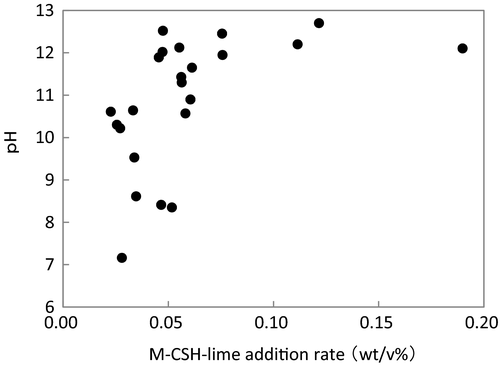
These results suggested that simultaneous color and 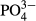 -P removal and disinfection of biologically treated wastewater from a swine farm were possible at M-CSH-lime addition rates of over 0.055 wt/v%.
-P removal and disinfection of biologically treated wastewater from a swine farm were possible at M-CSH-lime addition rates of over 0.055 wt/v%.
Performance of ADS reactor
Table 2 shows the water quality in the treatment process of the ADS reactor. The NO3−-N concentration was between 19.8–284.2 mg/L (average 161.1 mg/L) in the influent and 3.7–234.7 mg/L (average 127.3 mg/L) in the effluent. The average removal rate was 30.9%. NO2−-N was not detected in either the influent or effluent.
The NOx-N removal rate was naturally low in the initial stage of the experiment because the sulfur-denitrifying bacteria had not sufficiently accumulated. This rate increased considerably (approximately 70%) by approximately day 21, probably due to the growth of bacteria. Subsequently, the rate fluctuated depending on the changes in NOx-N loading rate and water temperature.
The removal rates under various loading rates are shown by Figure 6. The removal rate tended to increase as the loading rate decreased, and it increased to approximately 80% at a low loading rate of approximately 0.1 kg-N/ton-S/day despite a low water temperature of < 10°C. In general, the activity of sulfur-denitrifying bacteria is suppressed when the water temperature is < 10°C (Teshima & Mizuta 2009; Hasegawa et al. 2013; Hasegawa & Tanaka 2015b). Thus, denitrification activity would improve more when water temperature increases > 10°C.
The average  -S concentration increased from 32.8 mg/L (range, 26.9–42.8 mg/L) in the influent to 114.0 mg/L (range, 49.3–454.2 mg/L) in the effluent (Table 2). As shown in Figure 7, a good correlation was observed between the decrease in NOx-N (ΔNOx-N) and increase in
-S concentration increased from 32.8 mg/L (range, 26.9–42.8 mg/L) in the influent to 114.0 mg/L (range, 49.3–454.2 mg/L) in the effluent (Table 2). As shown in Figure 7, a good correlation was observed between the decrease in NOx-N (ΔNOx-N) and increase in 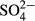 -S (Δ
-S (Δ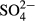 -S). This correlation indicates that sulfur consumption for denitrification was approximately 1.8 kg of sulfur per 1 kg of denitrified nitrogen. This ratio almost coincided with the theoretical value reported by Sierra-Alvarez et al. (2007) as almost all plots were located within the range of 95% confidence intervals. On the other hand, organic matter would not have contributed to denitrification because the influent BOD was considerably low. This suggests that only sulfur denitrification would contribute to a decrease in NOx-N in the ADS reactor.
-S). This correlation indicates that sulfur consumption for denitrification was approximately 1.8 kg of sulfur per 1 kg of denitrified nitrogen. This ratio almost coincided with the theoretical value reported by Sierra-Alvarez et al. (2007) as almost all plots were located within the range of 95% confidence intervals. On the other hand, organic matter would not have contributed to denitrification because the influent BOD was considerably low. This suggests that only sulfur denitrification would contribute to a decrease in NOx-N in the ADS reactor.
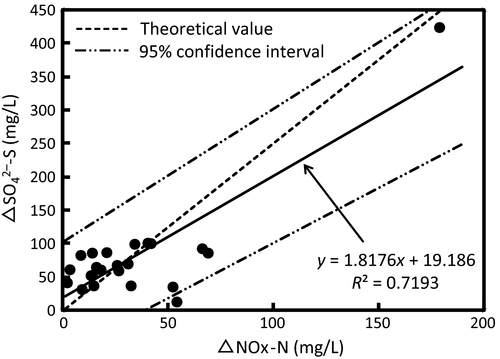
 -S.
-S.pH fluctuated from 7.1 to 9.4 in the influent and from 7.1 to 8.1 in the effluent after the expression of denitrification activity (after day 30). pH of the influent, which was alkalinized with M-CSH-lime, was neutralized by the ADS process. The pH level of this effluent is not an issue for discharge into public water bodies.
Characteristics of recovered M-CSH-lime
Table 3 shows the nutrient contents of the recovered M-CSH-lime and separated liquid from a flexible container bag in the recovery period from 27 November, 2014 to 10 December, 2014. The T-P2O5 content in the recovered M-CSH-lime was 9.22%. On the other hand, the nutrient contents in the separated liquid were hardly detected because most nutrients were collected in the bag. It was suggested that an easy recovery of used M-CSH-lime is enabled by the use of the bag.
| T-P2O5 (%) | T-N (%) | T-K2O (%) | T-MgO (%) | |
|---|---|---|---|---|
| Recovered M-CSH-lime | 9.22 | 0.26 | 0.27 | 2.57 |
| Separated liquid | Tr | 0.04 | 0.08 | Tr |
- Recovering period, 27 November, to 10 December, 2014. M-CSH-lime, modified amorphous silica and hydrated lime; T, total; Tr, trace.
Figure 8 shows the relationship between the M-CSH-lime/ -P ratio of the influent and T-P2O5 content in the recovered M-CSH-lime. It was shown that the T-P2O5 content in the recovered M-CSH-lime increased as the M-CSH-lime dose decreased. The maximum T-P2O5 content was 10.3%.
-P ratio of the influent and T-P2O5 content in the recovered M-CSH-lime. It was shown that the T-P2O5 content in the recovered M-CSH-lime increased as the M-CSH-lime dose decreased. The maximum T-P2O5 content was 10.3%.
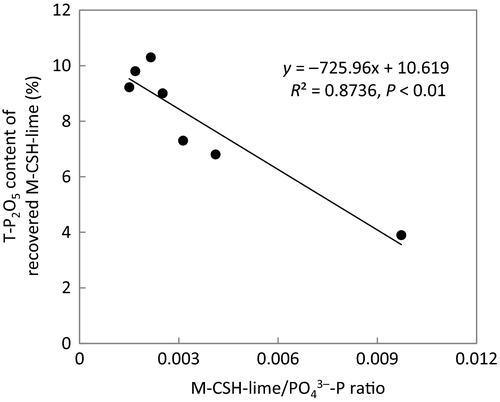
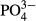 -P ratio of influent and T-P2O5 content in recovered M-CSH-lime.
-P ratio of influent and T-P2O5 content in recovered M-CSH-lime.Discussion
The advanced treatment of swine wastewater with M-CSH-lime was proven to be effective in a demonstration-scale treatment plant. An M-CSH-lime addition rate of approximately 0.055 wt/v% was sufficient for treatment; this addition rate was far lower than the rate of 0.15 wt/v% required in the bench-scale test (Tanaka et al. 2014; Hasegawa et al. 2014a). This difference would be related to the difference in the influent SS concentration. The influent SS concentration in the bench-scale test was 372 mg/L on average (Tanaka et al. 2014) and was significantly higher than that in the present study (< 3.2 mg/L). It was assumed that the treatment efficacy of M-CSH-lime was inhibited by high SS concentration, probably because of the coagulation of free lime and SS. Therefore, it is important to maintain low SS concentrations before M-CSH-lime treatment.
A high denitrification performance was observed when the NOx-N loading rate was approximately 0.1 kg-N/ton-S/day at an average water temperature of 14.6°C. The performance would be further increased at a water temperature above 15°C (Hasegawa & Tanaka 2015b).
To maintain sufficient denitrification activity throughout the year, heating the ADS reactor during the low temperature season should be indispensable. As a countermeasure, we have developed a simple heating system for reactors in swine farms (Hasegawa & Tanaka 2015b). The system consists of a stainless steel flexible tube, which is immersed into an aeration tank of activated sludge, and a submerged pump installed in the ADS reactor, which circulates the water of the reactor through the tube. In general, heat from the aeration tank is effectively transferred to the reactor by this system because the temperature of the aeration tank is rather higher than the ambient temperature during the low water temperature season. The denitrification performance would be improved using this heating system in the reactor.
Treatment process comprising M-CSH-lime followed by sulfur denitrification has the following two advantages. First, an ADS reactor may contribute to neutralization of the alkaline effluent from an M-CSH-lime reactor because of the formation of sulfate ions from sulfur. The sulfate ions in the effluent are not subject to regulation by the water pollution control law. However, it would be important to not discharge the effluent into a polluted pond because of the possibility of sulfide odor emission due to sulfate reduction. Second, CO2 gas purged into the effluent from the M-CSH-lime reactor for neutralization would serve as the inorganic carbon source for growth of sulfur-denitrifying bacteria. As reported by Hasegawa and Tanaka (2015a), high removal efficiency exceeding 78% can be obtained when the IC concentration was 230.3 ± 143.1 mg/L. In the present study, the IC concentration in the effluent before neutralization with CO2 gas was 24.5 ± 19.7 mg/L, which was considerably lower than the above value reported by Hasegawa and Tanaka (2015a). It was suggested that sufficient denitrification would not progress if CO2 gas had not been purged into the effluent. The sulfur denitrification occurred without the addition of calcium carbonate, which is generally indispensable for other ADS processes. This may verify the contribution of M-CSH-lime to the sulfur denitrification process.
The recovered M-CSH-lime should be valuable as a phosphorus resource. It could be used as chemical fertilizer because it contains calcium, potassium and magnesium in addition to phosphorus. Use of the recovered material in combination with solid compost of animal manure may also be an option for its effective utilization.
The recovered M-CSH-lime slurry would also be applicable as a binder for pelletizing dry powder compost made of animal manure. Pelletizing compost is an effective way of improving the value of the compost (Hasegawa & Tanaka 2014b). Animal manure compost is an important material for the maintenance and promotion of soil fertility, although the use of the compost is not promoted because its powdery composition makes it difficult to handle. Improving the compost handling ability is particularly needed in Japan. A molding technique for pelletizing compost is known, but the initial cost of the pellet molding apparatus is high. We have examined a technique for pelletizing compost that uses a simple concrete mixer and recovered M-CSH-lime slurry as a binder (Hasegawa & Tanaka 2014b). Granulated compost that is 5–30 mm in diameter could be successfully prepared by this technique. The prepared granulated compost, which contains fertilizer substances derived from the recovered M-CSH-lime, may improve the value of the compost.
Table 4 shows a cost estimate of the present treatment plant with M-CSH-lime and sulfur. When the quantity of waste water to be treated was 50 m3/day, the initial cost of the present treatment plant was 75 200 USD. On the other hand, the estimated initial cost of a treatment process that combined: (i) ozonation for decoloration; (ii) magnesium ammonium phosphate (MAP) for phosphorus removal; and (iii) methanol denitrification for nitrogen removal, was 440 000 USD. In addition, the running cost was 59 USD/day with the present treatment plant and 130 USD/day with prior plants. This estimation suggests that the present treatment plant is more economical than the prior plants.
| Treatment plant with M-CSH-lime and sulfur | Combinations of prior plants (Ozonation, MAP and methanol denitrification) | |
|---|---|---|
| Quantity of water to be treated | 50 m3/day | 50 m3/day |
| Initial cost | 75 200 USDa | 440 000 USDa |
| Running cost | 59 USD/day | 130 USD/day |
| Color removal rate | 78.0% | 80.0% |
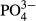 -P removal rate -P removal rate |
> 99.9% | 70.0% |
| NOx-N removal rate | 80.0% | 80.0% |
- a 1 USD (US dollar) = 100 yen (Japanese yen). M-CSH-lime, modified amorphous silica and hydrated lime; MAP, magnesium ammonium phosphate.




