Influence of riders’ skill on plasma cortisol levels of horses walking on forest and field trekking courses
Abstract
The aim of this study was to evaluate the influence of rider's skill on the plasma cortisol levels of trekking horses on two courses, walking on field and forest courses (about 4.5 to 5.1 km each). Three riders of different skills did horse trekking (HT) in a tandem line under a fixed order: advanced-leading, beginner-second and intermediate-last. A total of six horses were used and they experienced all positions in both courses; a total of 12 experiments were done. Blood samples were obtained before HT, immediately after and 2 h after HT. As a control, additional blood samples were obtained from the same horses on non-riding days. Irrespective of the course and the rider's skill, the cortisol level before HT was higher than that of control (P < 0.05). In both courses, the cortisol levels immediately after HT ridden by the advanced rider were higher than that of control (P < 0.05). However, in every case, the cortisol level 2 h after HT was closely similar to the level of the control. Thus, we concluded the stress of trekking horse was not sufficient to disturb the circadian rhythm of the cortisol level, irrespective of the course and the rider's skill.
Introduction
Horses have been used mainly for riding or draft purposes since they were domesticated. Human beings have clarified demands on horses and investigated them in order to maximize the capacity of horses from a viewpoint of animal science. When the horse is used for riding or draft purposes, power, speed and endurance are demanded of it. However, such roles of the horse were replaced by the machine with the rapid expansion of motorization in the 20th century. Accordingly, the main purpose of horse riding has changed from a means of transport to a popular sport and a hobby. In recent years, human beings also have found a new value in the horse. Even in France, in which orthodox dressage composes the major portion of riding, only the trekking population has been increasing. Amateur riders often do not expect a painful experience but pleasure. In other words, they often ride horses not for competition but for recreation or relaxation. As a result, the generality of riders prefer horse trekking (HT), also known as trail riding, as opposed to dressage in an arena. There are other statistics that suggest the HT population in Hokkaido, the spearhead in the rise of HT in Japan, may have increased more than 10 times from 1991 to 2006.
HT means riding along a walking trail in a forest, field or on a sandy beach. It is a popular leisure activity because it can generally be enjoyed regardless of age, sex and riding skill. Matsuura et al. (2011) showed that 30 min of HT with a walking gait strongly increased the parasympathetic nervous activities, improved mood and reduced mental fatigue of the rider. In recent times, 60 min of HT with trotting or walking gaits has been reported to shift the autonomic nervous system balance of experienced riders toward parasympathetic dominance (Matsuura et al. 2017). These studies indicate that HT has positive effects on human health. Given the results of these studies and the changes of the role of the horse, we can understand that leisure horses provide us with improvement in human health benefits and quality of life in addition to power, speed and endurance.
Usually in HT, horses are required to exercise outside the riding facilities where the horses are kept. Therefore, HT in an unfamiliar environment for the horses, including paved roads, steep slopes, and the sight and sound of approaching cars, motor bikes, bicycles and animals such as crows or stray cats and dogs, would be stressful. As horses are essentially nervous animals, related to their history as prey, encountering something strange on the trekking road might scare the horse and could make the horse show avoidance behaviors such as jumping or kicking while the rider is mounted. This could be a great hazard for the rider. On the other hand, HT might become a critical issue from the viewpoint of animal welfare, if horses are receiving such psychological loads frequently through HT. Therefore, understanding the stress of horses during HT is important for the safety of the riders and the welfare of the horses.
Although a lot of studies have investigated the stress experienced by horses caused by high-intensity treadmill exercise (Snow et al. 1992; Gordon et al. 2007) and galloping on a track (Kędzierski et al. 2013), few studies have examined the stress experienced by horses during HT. Medica et al. (2010) investigated the combined effect of trekking exercise and preliminary transport on adrenocortical and hematochemical responses in horses during trekking. Matsuura et al. (2010) studied the autonomic nervous activity of horses in the resting state after HT to compare stress responses between the leading and following horses in an HT tandem line. However, to date, no studies have examined the effect of the skills of the rider on the stresses experienced by horses. In addition, it is important to understand the effect of different courses on the stresses experienced by horses. In the present study, the plasma cortisol level was used as an indicator of stress in horses. The stress responses in horses that walked along the field and the forest trekking courses were compared based on the different skills of the riders, that is, beginner versus intermediate versus advanced.
Materials and methods
The animal experimentation protocol adopted in the present study was approved by the President of Kitasato University through judgment by the Institutional Animal Care and Use Committee of Kitasato University.
Horses, riders and experimental courses
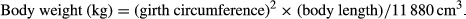
All horses were adequately acclimatized to the trekking course and owned by Towada Riding Club (Towada, Aomori Prefecture, Japan). Three adult male riders (beginner, intermediate and advanced) rode the horses. The riding skill of the beginner was at a level that he was able to trot, whereas that of the intermediate rider was at a level where he was able to participate in show jumping and dressage in domestic competitions. The advanced rider was an instructor certified by the National Riding Club Association of Japan.
The HT was conducted along two experimental trekking courses, the field course and the forest one. The total length of the field course was 5167 m, with a difference in altitude of 54 m. The total length of the forest course was 4553 m (2948 m in field and 1605 m in forest), with a difference in altitude of 108 m. The total length of the courses and the differences in altitude were measured using a global positioning system (Oregon 450 TC; Garmin Corporation, Taipei, Taiwan). The topographic maps of the trekking courses are shown in Figure 1.
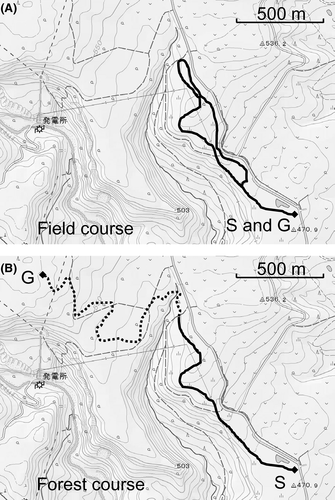
Experimental design
The influence between three riders’ skills were compared in two trekking courses. Three levels of the riders’ skills were advanced, intermediate and beginner. Two trekking courses were the field course and the forest one. Each HT was conducted using three horses in a tandem line (Horses A, B, C or D, E, F). The advanced, beginner and intermediate riders rode on the leading, second and last horses, respectively. The order of horse-rider pair in a tandem line of HT and the location of blood sampling are shown in Table 1. A total of six HT were done in the field course on separate days around the same time (details are given in the next section). Similarly, a total of six HT were done on the forest course on separate days around the same time.
| Experiment | Order (horse-rider) | Location of blood samplinga | ||||
|---|---|---|---|---|---|---|
| Leading | Second | Last | 10.00 hours | 11.30 hours | 13.30 hours | |
| Field course | ||||||
| # 1 | A-Advanced | B-Beginner | C-Intermediate | S (= G) | S (= G) | S (= G) |
| # 2 | B-Advanced | C-Beginner | A-Intermediate | S (= G) | S (= G) | S (= G) |
| # 3 | C-Advanced | A-Beginner | B-Intermediate | S (= G) | S (= G) | S (= G) |
| # 4 | D-Advanced | E-Beginner | F-Intermediate | S (= G) | S (= G) | S (= G) |
| # 5 | E-Advanced | F-Beginner | D-Intermediate | S (= G) | S (= G) | S (= G) |
| # 6 | F-Advanced | D-Beginner | E-Intermediate | S (= G) | S (= G) | S (= G) |
| Forest course | ||||||
| # 7 | A-Advanced | B-Beginner | C-Intermediate | S | G | G |
| # 8 | B-Advanced | C-Beginner | A-Intermediate | S | G | G |
| # 9 | C-Advanced | A-Beginner | B-Intermediate | S | G | G |
| #10 | D-Advanced | E-Beginner | F-Intermediate | S | G | G |
| #11 | E-Advanced | F-Beginner | D-Intermediate | S | G | G |
| #12 | F-Advanced | D-Beginner | E-Intermediate | S | G | G |
- a Saddles were visible to the horses at the sampling point. G, goal; S, start.
Procedure
Plasma cortisol is known to show a circadian rhythm, with a peak at 06.00–09.00 hours and a nadir at 18.00–21.00 hours (Irvine & Alexander 1994). For this reason, all measurements were performed between 09.00 and 13.30 hours in an effort to reduce the effect of such diurnal variation on the responses, as described by Jimenez et al. (1998).
Before HT, three horses were hitched to the posts at a wash rack at the course start point at 10.00 hours. Before the first blood sampling, saddles were visible to the horses although they were not saddled. After the first blood sampling, the experimenter initiated the saddling of each horse, finished by 10.25 hours. The HT was performed with a walking gait from 10.30 to 11.30 hours along the field or forest course. Each horse underwent HT three times in the field course and three times in the forest course. After HT (at 11.30 hours), each rider dismounted his horse and hitched the horse to either the posts at the wash rack (field course) or a tree (forest course). The horses were held for 120 min to maintain a resting state. Blood samples were obtained from the jugular veins of all horses before HT, immediately after HT and 2 h after HT. As a control, blood was sampled from the same six horses under the same schedule on non-riding days at the start point (Table 2). The saddles were not visible to the horses in control sampling. The time schedule of blood sampling is shown in Figure 2.
| Experiment | Horse | Location of blood samplinga | ||||
|---|---|---|---|---|---|---|
| 10.00 hours | 11.30 hours | 13.30 hours | ||||
| Control | ||||||
| # 1 | A | B | C | S (= G) | S (= G) | S (= G) |
| # 2 | D | E | F | S (= G) | S (= G) | S (= G) |
- a Saddles were not visible to the horses at the sampling point. G, goal; S, start.
Cortisol assay
Blood samples were collected in tubes containing heparin, and plasma was separated after centrifugation (2000 × g, 10 min) and placed on ice. Within 2 h, the plasma was stored at −30°C. Plasma cortisol levels were analyzed in duplicates using the Cortisol Express EIA kit (Cayman Chemical Inc., Ann Arbor, MI, USA) according to the manufacturer's protocol. The plasma samples were diluted to 1:10. For technical reasons, 36 of the 126 samples and the remaining 90 samples were separately assayed. The average intra- and inter-assay coefficients of variation for 36 samples were 2.38% and 8.27%, respectively. The average intra- and inter-assay coefficients of variation for 90 samples were 2.20% and 10.77%, respectively.
Statistical analysis
Data are presented as mean ± SE. Data were analyzed by using the one-way repeated measures analysis of variance (ANOVA) followed by Dunnet's multiple comparison test. Statistical significance was denoted by a comparison with the control (10.30 hours). Differences were considered to be significant at P < 0.05.
Results
Body measurements and estimated body weights of the horses are shown in Table 3. Withers height (mean ± SD) was 138.3 ± 6.78 cm, body length was 149.3 ± 12.22 cm, girth circumference was 164.5 ± 9.47 cm, hip length was 48.0 ± 2.83 cm, cannon circumference was 17.5 ± 0.99 cm and estimated body weight was 343.6 ± 66.03 kg.
| Horse | Sex | Age (years) | Body measurement | |||||
|---|---|---|---|---|---|---|---|---|
| Height at withers (cm) | Body length (cm) | Girth circumference (cm) | Hip length (cm) | Cannon circumference (cm) | Estimated body weighta (kg) | |||
| A | G | 7 | 149.6 | 161.5 | 177.2 | 50.0 | 18.8 | 426.9 |
| B | M | 25 | 143.7 | 161.1 | 172.7 | 51.1 | 18.5 | 404.4 |
| C | G | 7 | 132.8 | 134.0 | 155.1 | 46.0 | 17.1 | 271.3 |
| D | M | 7 | 134.3 | 151.0 | 166.5 | 47.0 | 17.5 | 352.4 |
| E | M | 6 | 135.7 | 153.0 | 162.3 | 50.0 | 17.2 | 339.2 |
| F | G | 7 | 133.8 | 134.9 | 153.4 | 43.8 | 16.1 | 267.2 |
- a Body weight of each horse was estimated from the following formula according to Wagner and Tyler (2011): body weight (kg) = (girth circumference)2 × (body length)/11 880 cm3. G, gelding; M, mare.
Figure 3 shows the plasma cortisol levels of the horses during exercise along the field trekking course in comparison with the level of the control (10.30 hours). The cortisol levels of horses (mean ± SE, ng/mL) ridden by the beginner (before HT: 7.29 ± 1.06), intermediate (before HT: 6.85 ± 1.11) and advanced (before: 8.10 ± 0.69 and immediately after HT: 8.18 ± 2.19) riders were higher (P < 0.05) in comparison with the level of the control at 10.30 hours (5.38 ± 1.29). Focusing on before HT, the cortisol levels on riding days were significantly higher than that of non-riding control days (P < 0.05), irrespective of rider's skill. Focusing on immediately after HT, the cortisol levels in the mount of the advanced rider was significantly higher than that of control before HT (P < 0.05). Focusing on 2 h after HT, the cortisol levels (mean ± SE, ng/mL) on riding days (2.80 ± 0.19) were similar to that of non-riding days (2.99 ± 0.27).
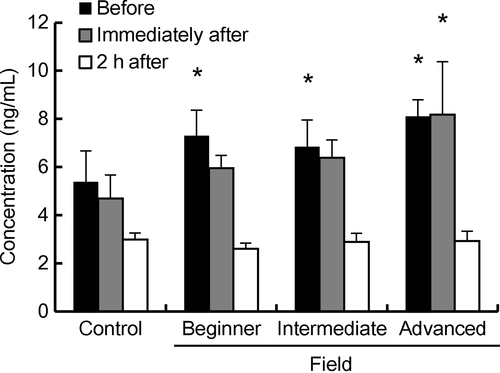
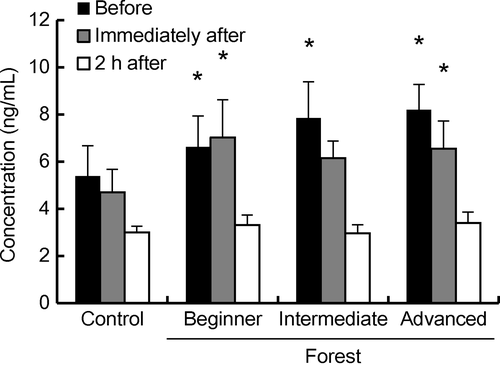
Figure 4 shows the plasma cortisol levels of the horses during exercise along the forest trekking course in comparison with the level of the control at 10.30 hours. The cortisol levels of horses (mean ± SE, ng/mL) ridden by the beginner (before: 6.63 ± 1.31 and immediately after HT: 7.03 ± 1.59), intermediate (before HT: 7.85 ± 1.53) and advanced (before: 8.20 ± 1.07 and immediately after HT: 6.55 ± 1.17) riders were higher (P < 0.05) in comparison with the level of the control at 10.30 hours (5.38 ± 1.29). Focusing on before HT, the cortisol levels on riding days were significantly higher than that of non-riding control days (P < 0.05), irrespective of rider's skill. Focusing on immediately after HT, the cortisol levels in the mount of the beginner and the advanced rider were significantly higher than that of control before HT (P < 0.05). Focusing on 2 h after HT, the cortisol levels (mean ± SE, ng/mL) on riding days (3.21 ± 0.23) were similar to that of non-riding days (2.99 ± 0.27).
Discussion
Irrespective of trekking course and rider's skill, the cortisol level before HT was significantly higher than that of the control at 10.30 hours (P < 0.05). Although the cortisol level has been reported to increase after exercise (Marc et al. 2000; Kędzierski et al. 2013), the present study is the first to show an increase in the level before exercise. In a study by Parrott et al. (1994), plasma cortisol levels were significantly increased for sheep standing in a pen filled with tap water, the oscillating pen of a transport simulator and isolation. The significant increase in cortisol level before HT observed in the present study could be ascribed to the mental stress of the horses because of anticipation of being ridden; in the present study, the saddle was within sight of each horse during blood sampling before HT, although the experimenter did not saddle each horse at that time. It is likely that the study horses have learned to associate the saddle with a high likelihood of being ridden. This result suggests that the rider should mitigate the stress of the horse, especially prior to mounting, at the moment of mounting and at the start of HT, for example prior to mounting, it is desirable that the rider builds a good relationship with the horse by greeting and touching the horse. At the moment of mounting, the rider should talk gently to the horse and mount smoothly so as not to apply excessive pressure on the back. Before starting HT, the rider and horse would be better to exercise in a familiar place together with other horses. Touching the proximal region of the crest of the horse would also be effective.
High cortisol levels in the mount of the advanced rider after HT at 11.30 hours in both courses (P < 0.05) could be due to the fact that the advanced rider rode on the leading horse. The stress experienced by the leading horse has already been reported to be larger than that experienced by the following horse (Matsuura et al. 2010). In free-range horses, an older or larger mare is apt to be the highest ranking female, and it is she who leads the herd in flight and in daily journeys to rest or to a new grazing area (Houpt 1998). Also in Przewalski horses (Equus przewalskii), the dominant mares control the direction of movement during pasturing, migration and flight from danger (Klimov 1988).
Therefore, the horse that took a leading role would be relatively constant under natural conditions. If the order of which the horse leads the herd is changed, it will lead to confusion and stress in the herd. In contrast to free-range behavior, the horse chosen to lead the pack in an HT single file would not necessarily be the leader, namely the horse with the most ability to withstand various types of stressors. During HT, the order of the horses in tandem line is decided in light of various factors, for example skill and physique of the rider, endurance and speed of the horse. Consequently, the position adopted by a horse in tandem line along an HT course frequently changes. In consideration of the discrepancy between the behavior of wild horses and the horses undergoing HT, we speculated that high cortisol levels in the mount of the advanced rider was derived from being positioned out of the natural dominance order of the herd, and the effect will be larger on the leading horse than on those following.
The cortisol level of the horse ridden by the beginner immediately after HT was higher than that of the control at 10.30 hours only in the forest course, but not in the field course. According to Terada (2000), studying a novice rider showed a frequency distribution dispersion of the rider's head in the proximal–distal direction which was not observed in the advanced rider. The result of the present study suggests that the beginner rider imposed a burden on the horse because it was difficult to maintain its balance in the forest course. Another remarkable finding is that the cortisol level 2 h following HT at 13.30 hours (3.01 ng/mL) dropped to the level of the control (2.99 ng/mL), irrespective of trekking course and rider's skill. The circadian rhythm of the plasma cortisol level resulted in a decrease during this period (Irvine & Alexander 1994; Giannetto et al. 2014). Thus, the stress levels incurred under this experimental condition were not sufficient to disturb the circadian rhythm. In other words, the stress dissipated 2 h following HT, while the horses were walking.
In conclusion, the cortisol level before HT was high, irrespective of trekking course and rider's skill. Therefore, we suggest that the rider should mitigate the stress of the horse, especially prior to mounting, at the moment of mounting and at the beginning of HT. However, the stress levels experienced by the horse are not sufficient to disturb the circadian rhythm of the cortisol level, irrespective of trekking course and rider's skill. A limitation of the present study was that the positions of the horses on the trail were not counterbalanced. Although the experienced rider went first for safety reasons, we could evaluate three riding levels balanced with a fourth experienced rider. Further studies are also needed to understand the stress responses of horses during more practical HT in which the horse will trot and canter, also using behavioral indices. However, the outcome of the present study could improve safety of the riders and the welfare of the leisure horse.
Acknowledgments
This study was supported financially by JSPS KAKENHI Grant Number 26450393.




