Involvement of circadian clock in crowing of red jungle fowls (Gallus gallus)
Abstract
The rhythmic locomotor behavior of flies and mice provides a phenotype for the identification of clock genes, and the underlying molecular mechanism is well studied. However, interestingly, when examining locomotor rhythm in the wild, several key laboratory-based assumptions on circadian behavior are not supported in natural conditions. The rooster crowing ‘cock-a-doodle-doo’ is a symbol of the break of dawn in many countries. Previously, we used domestic inbred roosters and showed that the timing of roosters' crowing is regulated by the circadian clock under laboratory conditions. However, it is still unknown whether the regulation of crowing by circadian clock is observed under natural conditions. Therefore, here we used red jungle fowls and first confirmed that similar crowing rhythms with domesticated chickens are observed in red jungle fowls under the laboratory conditions. Red jungle fowls show predawn crowing before light onset under 12:12 light : dim light conditions and the free-running rhythm of crowing under total dim light conditions. We next examined the crowing rhythms under semi-wild conditions. Although the crowing of red jungle fowls changed seasonally under semi-wild conditions, predawn crowing was observed before sunrise in all seasons. This evidence suggests that seasonally changed crowing of red jungle fowls is under the control of a circadian clock.
Introduction
The circadian system of animals synchronizes daily timing of activity with the environmental light–dark cycle. The rhythmic locomotor behavior of flies and mice provides phenotypes for the identification of clock genes (Gallego & Virshup 2007; Hardin 2011), and the underlying molecular mechanism is well studied (Gerstner & Yin 2010). For example, under laboratory light–dark cycles, flies enhanced locomotor activity before light signals (Helfrich-Forster 2001; Collins et al. 2005), and these anticipatory behaviors have defined the neuronal sites of the corresponding morning and evening oscillators (Grima et al. 2004; Stoleru et al. 2004). However, interestingly, when examining locomotor rhythm in the wild, several key laboratory-based assumptions on circadian behavior are not supported in natural conditions (Vanin et al. 2012).
The rooster crowing ‘cock-a-doodle-doo’ was used to inform the ancients of the Indus civilization (B.C. 2600–1800) of the start of day (Peake & Fleure 1931, 1933) and remains a symbol of the break of dawn in many countries. However, until recently, it remained unclear whether crowing is under the control of an internal biological clock or is simply caused by an external stimulus, such as the sunrise. Previously, we used domestic inbred roosters and showed that the timing of roosters' crowing is regulated by the circadian clock under laboratory conditions (Shimmura & Yoshimura 2013). The anticipatory predawn crowing was observed before the onset of light under 12:12 h light : dim light conditions, and a free-running rhythm of crowing was observed under constant dim light conditions (Shimmura & Yoshimura 2013). However, it is still unknown whether the regulation of crowing by circadian clock is observed under natural conditions.
Jungle fowls (Gallus gallus) are the ancestors of domestic chickens. In order to improve their productivity as livestock, chickens have been domesticated from jungle fowls over the course of several thousand years (Miao et al. 2013). Today, highly domesticated chickens lay more than 300 eggs through the year, whereas red jungle fowls exhibit seasonal response (Ono et al. 2009). Therefore, here we used red jungle fowls and first show that similar crowing rhythms with domesticated chickens are observed in red jungle fowls under laboratory conditions (Experiment 1). We also show that seasonally changed crowing of red jungle fowls is under the control of a circadian clock under semi-wild conditions (Experiment 2).
Materials and Methods
Animals and experimental environment
Fully matured red jungle fowl (Gallus gallus; one year of age or more) roosters that exhibited crowing behavior were used for the experiments. All roosters were raised in pens. The roosters had ad libitum access to water and feed. Animals were treated in accordance with the guidelines of Tokai University. All experimental protocols were approved by Tokai University (#131112).
Experiment 1 (Indoor experiment)
Indoor experiments were conducted in a light-tight room (Tokai University). Three roosters were kept in individual cages (44 cm × 61 cm × 65 cm) to reduce the risk of mortality of lower-ranking roosters due to interactions with aggressive higher-ranking roosters.
Experiment 2 (Outdoor experiment)
Outdoor experiments were conducted in semi-wild conditions (Kumamoto City Zoo). Each group consisted of one rooter and three hens. A total of four roosters (four groups) were examined. These groups were kept in large cages (500 cm × 250 cm × 300 cm). The cages were separated by wire mesh that allowed roosters to recognize each other visually and aurally.
Behavioral observation
Rooster crowing was recorded all day using a digital video camera equipped with a near-infrared illuminator (DCR-SR65 and DCR-SR220, Sony) or an IC recorder (VN-722PC, Olympus). Because red jungle fowl roosters exhibit elevation and extension of the head prior to and during crowing, the crowing of particular roosters is distinguishable (Shaw & Kennedy 2002), and therefore the crowing of each individual rooster could be easily distinguished from the video and sound recordings.
Experiment 1 (Indoor experiment)
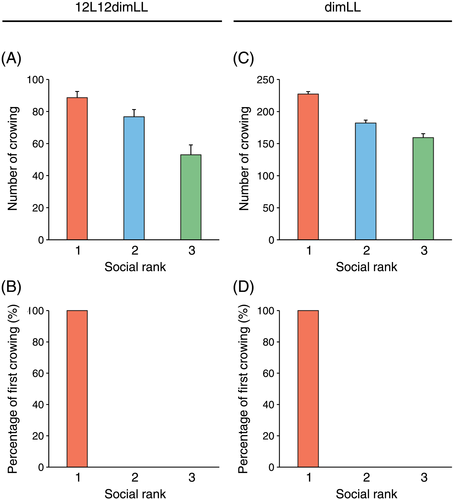
In the indoor experiments, all roosters were kept under 12-h light : 12-h dim light (12L12dimL) conditions for 10 days to allow adaptation before the beginning the experiment. To test whether crowing of red jungle fowls is under the control of an internal biological clock (i.e. circadian clock), or is instead controlled by external stimuli such as light, all roosters were kept under 12L12dimL cycles for 10 days, and then under constant dim light (dimLL) condition for 10 days. Lighting was adjusted to give an intensity of 100 lux (regular light) or < 1 lux (dim light) at the height of the roosters' heads. When one rooster breaks the dawn, others in the neighborhood have a higher probability of crowing (Koene 1996). Therefore, we also examined the social interactions between roosters in regard to crowing. Because red jungle fowls have high aggressiveness, it was difficult to determine the social rank by introducing them into a group cage. Therefore, we determined social rank by the number of crows (Fig. 2A), because crowing is an androgen-dependent behavior and is one of the best-known indices of social hierarchy (Salomon et al. 1966; Shimmura et al. 2015).
Experiment 2 (Outdoor experiment)
In the outdoor experiments, as shown previously in birds (Follett & Sharp 1969; Ikegami et al. 2014), red jungle fowls laid more eggs in the spring (0.43 ± 0.06 eggs/hen/day) and fewer in the autumn (0.17 ± 0.04 eggs/hen/day). Therefore, two observations were conducted, one in spring (May and June), representing the breeding season, and the other in autumn (September), representing the non-breeding season. The average day length was 14.12 h in spring and 11.59 h in autumn. The sunlight fell into the cages. The light intensity of twilight before sunrise was 10 lux that chickens can recognize (Lewis & Morris 1999; Shimmura & Yoshimura 2013). We could not analyze the social interactions of Experiment 2 due to the small size of the dataset.
Statistical analysis
Periods of crowing under dimLL conditions were calculated by Lomb-Scargle periodogram analysis (Ruf 1999) using the ClockLab software v2.61 (Actimetrics, Evanston, IL, USA). The number of crowings in the breeding and non-breeding seasons were compared by paired t-test.
Results
Experiment 1 (Indoor experiment)
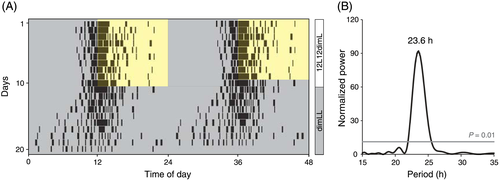
Under 12L12dimL cycles, crowing was observed on average 2.23 ± 0.45 h before the onset of light (i.e. anticipatory predawn crowing; Fig. 1A). Under dimLL conditions, a free-running rhythm of crowing was observed (Fig. 1A,B). The averaged free-running rhythm of crowing of three roosters was a period of 23.7 ± 0.1 h. As expected, higher-ranking roosters tended to crow more frequently than lower-ranking roosters under the 12L12dimL (Fig. 2A) and the dimLL (Fig. 2C) conditions. Under 12L12dimL cycles, the highest-ranking rooster always crowed first every morning (Fig. 2B). Consistent results were also observed in free-running predawn crowing under dimLL conditions (Fig. 2D).
Experiment 2 (Outdoor experiment)
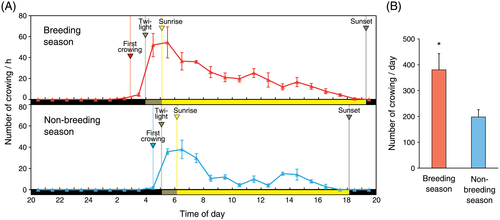
Red jungle fowls exhibited seasonal changes in crowing, and the number of crowings was higher in the breeding season than in the non-breeding season (Fig. 3A,B). However, red jungle fowls showed predawn crowing before sunrise in both seasons (Fig. 3A). The predawn crowing was observed before the start of twilight (Fig. 3A).
Discussion
Although the seasonal response of domestic chickens is robust (Wilson & Sharp 1975), Ono et al. (2009) reported that red jungle fowls, the predecessors of domestic chickens, exhibit a clear physiological response to seasonal changes. When red jungle fowls were transferred from short-day to long-day conditions, gonadal development and increases in plasma leutinizing hormone concentration were observed (Ono et al. 2009). Crowing is an androgen-dependent behavior (Berthold 1849; Marler et al. 1962), and exogenous androgen administration enhances crowing behavior (Shimmura & Yoshimura 2013). As expected, we observed in this study that red jungle fowls exhibited seasonal changes in crowing, and the number of crowings was higher in the breeding season than in the non-breeding season under semi-wild conditions.
More interestingly, the first crowing was observed before sunrise (i.e. anticipatory predawn crowing) in both breeding and non-breeding seasons. Although it is possible that chickens can recognize the twilight (10 lux) before sunrise (Lewis & Morris 1999; Shimmura & Yoshimura 2013), the anticipatory predawn crowing was also observed before the start of twilight. This result suggested the involvement of a circadian clock in crowing of red jungle fowls. In addition, we also confirmed in a light-tight room that the predawn crowing occurred before the onset of light under a light–dark cycle and that the free-running rhythm of crowing was observed under constant dim light. These results are completely consistent with our previous studies demonstrating the involvement of the circadian clock in morning crowing of domesticated inbred roosters (Shimmura & Yoshimura 2013; Shimmura et al. 2015). Therefore, our present observations indicate that seasonally changed crowing of red jungle fowls is under the control of a circadian clock.
When one rooster breaks the dawn, others in the neighborhood have a higher probability of crowing (Koene 1996). Previously, it was thought that there were no rules regarding when roosters crow relative to one another. However, our previous study demonstrated that the highest-ranking rooster always started to crow first, followed by lower-ranking roosters in descending order of social rank (Shimmura et al. 2015). Thus, in a group situation, the top-ranking rooster has priority to announce the break of dawn, and subordinate roosters are patient enough to wait for the top-ranking rooster's first crow every morning (Shimmura et al. 2015). Our present observations suggested that the social order of crowing also exists in red jungle fowls. Under wild conditions, each group of jungle fowls lives in separate areas and avoids direct aggressive interactions (Collias & Collias 1996), and each rooster's crowing serves to advertise its own group's territories (Collias & Collias 1967). Therefore, it may be reasonable to hypothesize that red jungle fowls use crowing to signal their territories in each rank, thereby reducing the risk of aggressive interactions.
In summary, in this study, we showed that red jungle fowls exhibited seasonal changes in crowing. However, the seasonally changed crowing is also under the control of a circadian clock.
Acknowledgments
We thank Dr. T. Yoshimura for technical support. This work was supported in part by JSPS Grant-in-Aid for Research Activity Start-up 25892013 and for Young Scientist (B) 15 K18802, by the Center for the Promotion of integrated Sciences (CRIPS) of SOKENDAI, and by the Cooperation Research Program of Wildlife Research Center of Kyoto University. The authors declare no competing financial interests.




