Ovarian responses of dairy buffalo cows to timed artificial insemination protocol, using new or used progesterone devices, during the breeding season (autumn–winter)
Abstract
This study evaluated the effect of new or used P4 devices on the ovarian responses of dairy buffalo that were administered an estradiol (E2) plus progesterone (P4)-based timed artificial insemination (TAI) protocol during the breeding season. On the first day of the TAI protocol, 142 cows were randomly assigned to receive one of the following: a new device (New; 1.0 g of P4; n = 48); a device that had previously been used for 9 days (Used1x, n = 47); or a device that had previously been used for 18 days (Used2x, n = 47). Ultrasound was used to evaluate the following: the presence of a corpus luteum (CL); the diameter of the dominant follicle (ØDF) during protocol; ovulatory response; and pregnancies per AI (P/AI). Despite similar responses among the treatments, there was a significant positive association of the ØDF during TAI protocol with ovulatory responses and number of pregnancies. In conclusion, satisfactory ovarian responses and a satisfactory pregnancy rate were achieved when grazing dairy buffalo were subjected to the TAI protocol in breeding season, independent of whether a new or used P4 device was used. Furthermore, the presence of the larger follicle was associated with a higher ovulation rate and higher P/AI following TAI.
Introduction
Artificial insemination (AI) is an important tool for improving the genetic gain progress in dairy herds. This biotechnology has been used in buffalo (Bubalus bubalis) during the breeding season (decreasing day length) upon estrus detection (Ohashi 1994) with a satisfactory number of pregnancies per AI (P/AI ≥ 40%) (Baruselli 1996). However, these results can be severely impacted because of the low homosexual behavior and discrete manifestation of estrus in buffalo (Baruselli et al. 1997; Zicarelli et al. 1997; Porto-Filho et al. 2014).
In contrast to this limited situation, the synchronization of ovulation protocols for timed AI (TAI) allows for AI without the need for estrus detection and at a predetermined time. In the breeding season (autumn and winter), satisfactory P/AI are achieved using the GnRH (buserelin acetate) plus PGF2α (sodium cloprostenol) based protocol in synchronized cyclic buffalo (Baruselli et al. 1999a,b, 2002; Campanile et al. 2010; Vecchio et al. 2012). However, the efficiency of this protocol is compromised when applied outside the breeding season (spring-summer) (Baruselli et al. 2003b). Estradiol (E2) plus progesterone (P4) TAI-based protocols have been successfully applied in lactating buffalo cows outside the breeding season (Baruselli et al. 2002, 2003a; Porto-Filho et al. 2004; Carvalho et al. 2005, 2007a,b). These reproductive programs are particularly important for the dairy buffalo industry because they avoid reliance on the natural calving seasonality, which ensures milk production throughout the year (Bernardes 2007; Carvalho et al. 2011, 2014; Monteiro et al. 2014).
In bovines, the P4 concentration during TAI protocols has a direct influence on the reproductive response by controlling luteinizing hormone (LH) pulsatility (Ireland & Roche 1982) and the ovulatory follicle size (Savio et al. 1993). Nevertheless, low P4 concentrations during synchronization of high-producing dairy cows can compromise their fertility due its impact on oocyte quality (Wiltbank et al. 2011a,b) and on the uterine receptivity for pregnancy (Cerri et al. 2011). However, high endogenous P4 concentrations (resulting from corpus luteum (CL) production plus exogenous P4 sources) compromise follicular growth and ovulation in cattle (Carvalho et al. 2008; Dadarwal et al. 2013; Barreiros et al. 2014). Similarly, in dairy buffalo, Mendes et al. (2014) described worse follicular responses with the E2 plus P4-based TAI protocol when buffalo were synchronized in the presence of a CL. Furthermore, the negative impact of the P4 concentration excess during dominant follicular growth could be particularly important when cyclic buffalo cows are synchronized with E2 plus P4 TAI protocols using a new P4 device during the breeding season. However, limited information about this situation has been reported.
In a recent study on the seasonal anestrus of dairy buffalo, Carvalho et al. (2014) showed that different P4 concentrations were released from new and previously used P4 devices (New – 2.5 ± 0.1 ng/mL; Used1x – 1.5 ± 0.0 ng/mL; and Used2x – 1.1 ± 0.0 ng/mL; P < 0.01). Despite these differences, similar follicular growth and ovulation and a satisfactory P/AI were reported. However, it is not known whether exogenous P4 source utilization associated with endogenous luteal P4 compromises dominant follicle development, ovulation and pregnancy rates in buffalo inseminated in the breeding season, when more than 70% of the buffalo have an active CL during the TAI protocol (Monteiro et al. 2014).
Thus, the aim of the present study was to evaluate the effect of new or used P4 devices on ovarian response in grazing buffalo cows submitted to TAI during the breeding season (autumn–winter). Our hypothesis is that during the breeding season, buffalo cows treated with new P4 devices present a worse ovarian outcome than do those treated with the used P4 devices.
Materials and Methods
Animals
This research (protocol number 2240/2011) agreed with Ethical Principles in Animal Research adopted by ‘Ethic Committee in the Use of Animal’ of the School of Veterinary Medicine and Animal Science of University of São Paulo and was approved in the meeting of 06/22/2011. The experiment was conducted in three commercial farms of Ribeira Valley located in Sao Paulo state, Brazil, during the breeding season (autumn–winter solstice in the southern hemisphere; from May to July). A total of 142 crossbreed multiparous buffalo (Murrah and Mediterranean) were used and maintained exclusively in Brachiaria decumbens pasture, with free access to water and mineral salt. Data on days in milk (DIM) and body condition score (BCS) of the females were recorded on the first day of the TAI protocol (Day 0). The BCS scale was from 1 to 5, where 1 corresponds to emaciated animals and 5 corresponds to obese animals (Baruselli et al. 2001).
Experimental design
On a random day of the estrous cycle, buffalo cows received the same TAI synchronization protocol (Fig. 1). On the first day of the synchronization (Day 0), females received 2 mg of estradiol benzoate (EB, Sincrodiol®, Ourofino Agribusiness, Cravinhos, Sao Paulo, Brazil) intramuscularly (IM) and an intravaginal P4 device (Sincrogest®, Ourofino Agribusiness, Cravinhos, Sao Paulo, Brazil). At this time, cows were randomly divided into three groups, each of which received one of the following: a new device (New, n = 48); a device that was previously used for 9 days (Used1x, n = 47); or a device that was previously used for 18 days (Used2x, n = 47). After use, the P4 devices were individually washed with water and then soaked in chloride alkyl dimethyl benzyl ammonium solution (CB30; Ourofino Agribusiness) for approximately 10 min. Thereafter, the P4 devices were dried using brown paper, placed inside the original new device bags, and stored at room temperature until their next use.
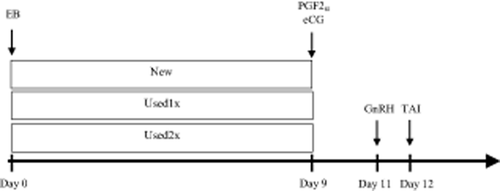
Synchronization of an ovulation protocol for timed artificial insemination (TAI) during the breeding season of grazing dairy buffalo cows. On the first day of the synchronization (Day 0), females received 2 mg of estradiol benzoate (EB) intramuscularly (IM) and a P4 device. At this time, the cows were randomly divided into one of three groups: a new P4 device (New P4, n = 48); a device that was previously used for 9 days (Used1x P4, n = 47); and a device that was previously used for 18 days (Used2x P4, n = 47). On Day 9, the P4 device was removed and 0.53 mg of sodium cloprostenol (PGF2α) and 400 IU of equine chorionic gonadotropin (eCG) were administered IM. The ovulation was induced 48 h after P4 device removal (Day 11), using 10 μg of buserelin acetate (GnRH) IM. The animals were inseminated 16 h after the GnRH administration (Day 12).
On Day 9, the P4 device was removed and 0.53 mg of PGF2α (Sincrocio®, Ourofino Agribusiness, Cravinhos, Sao Paulo, Brazil) and 400 IU of equine chorionic gonadotropin (eCG; Novormon®, MSD Animal Health, Sao Paulo, Sao Paulo, Brazil) were administered IM. Ovulation was induced 48 h after the P4 device removal (Day 11), using 10 μg of GnRH (Sincroforte®, Ourofino Agribusiness, Cravinhos, Sao Paulo, Brazil) IM. All animals were inseminated 16 h after the GnRH administration (Day 12). The inseminations were performed by the same technician using semen from a single sire with proven fertility in previous TAI programs.
Ultrasonographic evaluation
The females underwent ovarian follicular dynamic evaluation with a linear transducer of 5.0 MHz (Chison D600Vet, Shenzhen, China). The ultrasound examinations were performed by the same examiner, using the same settings and image resolutions. Cows were evaluated according to the following: cyclicity (a buffalo is considered cyclic in the presence of a CL at the P4 device implantation (CLD0) and/or removal (CLD9)); diameter of the dominant follicle (ØDF) at P4 device removal (Day 9); ØDF at TAI (Day 12); diameter of the CL 10 days after TAI (ØCL); daily growth rate of the dominant follicle (mm/day) calculated as the difference between the ØDF measured at TAI and the ØDF measured at P4 device removal (dividing by 2 days between the P4 device removal (Day 9) and the GnRH-induced ovulation (Day 11)).
The ovulation rate (Ov) was determined 10 days after TAI based on the presence or absence of a CL in the same ovary containing the DF on both Day 9 and Day 12 of the synchronization protocol. ‘Ovulation before AI’ occurred when the buffalo had dominant follicles ≥ 7 mm on D9 and a CL 10 days after TAI in the same ovary, without any follicle ≥ 5 mm present at AI (D12).
Pregnancy diagnosis was performed at 30 (P/AI 30 days) and 45 days (P/AI 45 days) after TAI, with the detection of a viable embryo compatible with these periods used as an indicator of pregnancy (No. of pregnant buffalo/No. of inseminated buffalo). The embryonic mortality (EM) was determined based on the number of cases in which the embryo or the embryo heartbeat was absent at 45 days after TAI was successful compared with the total number of females that had heart-beating embryos at 30 days (((No. of pregnant buffalo at 30 days − No. of pregnant buffalo at 45 day)/No. of pregnant buffalo at 30 days)*100).
Statistical analyses
Continuous variables were presented as the mean ± standard error of the mean (mean ± SEM) and percentage (%) for frequency of occurrence for binomial variables. The comparison among variables for each treatment (New, Used1x or Used2x) was performed by analysis of variance (ANOVA), using the GLIMMIX procedure of SAS® version 9.3 (SAS/STAT; SAS Institute Inc., Cary, NC, USA). The graphics were created using Sigmaplot 11 (Systat Software GmbH, Erkrath, Germany) software program. The tables were created using Microsoft Excel version 2010 for Windows.
The continuous response variables were subjected to the response scaling test through the Guided Data Analysis solution of SAS. Variables that did not follow these assumptions were transformed accordingly. Correlation analysis between all response variables was performed using the CORR RANK SAS procedure. Statistical models were formed by classificatory variables (treatment, farm, treatment*farm), continuous response variables (DIM, BCS, ØDF Day 9, ØDF Day 12, growth rate of DF and ØCL; dist = normal), binomial response variable (cyclicity, ovulation before AI, Ov, P/AI 30 days, P/AI 45 days and EM; dist = binomial) and linear effect of DIM, BCS and CLD9.
The relationships between the ØDF Day 9 and ØDF Day 12 with ovulation and pregnancy at 45 days were determined. Logistic regression curves were created using the coefficients provided by the interactive data analysis from SAS (Logit = intercept + slop*(ØDF)). The probability curves were obtained using the following formula: Y = (EXP(logit)/1 + EXP(logit))*100. An additional retrospective analysis was performed, which considered the presence or absence of a CL at P4 device removal (CLD9 or No-CLD9) in relation to the response variables. A significant difference was considered when P < 0.05.
Results
None of the evaluated variables showed an interaction effect between treatment and farm (P > 0.05). Independent of the New device, Used1x device or Used2x P4 device, the buffalo cows presented similar DIM (114.9 ± 17.5, 98.0 ± 10.2, 109.9 ± 14.2 d, respectively; P = 0.96) and BCS (3.1 ± 0.1, 3.3 ± 0.1, 3.2 ± 0.1, respectively; P = 0.31) at the beginning of the trial. In general, 80.2% of the buffalo (114/142) presented a CL at the insertion and/or at the removal of the P4 device, also without differences among the treatments (Table 1). The ØDF at P4 device removal (Day 9) and at the TAI (Day 12), the growth rate of DF, the ovulation rate before AI and the ØCL 10 days after AI were all not affected by treatment (Table 1, P > 0.05).
| Item | Treatment | P-values | ||
|---|---|---|---|---|
| New | Used1x | Used2x | ||
| No. of cows | 48 | 47 | 47 | – |
| Cyclicity (%) | 81.2 | 78.7 | 80.8 | 0.65 |
| ØDF at Day 9 (mm) | 9.0 ± 0.4 | 9.0 ± 0.3 | 9.6 ± 0.4 | 0.40 |
| ØDF at Day 12 (mm) | 12.2 ± 0.5 | 12.3 ± 0.3 | 12.6 ± 0.4 | 0.58 |
| Growth rate of DF (mm/day) | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 0.77 |
| Ovulation rate (%) | 77.1 | 87.2 | 82.9 | 0.69 |
| Ovulation before AI (%) | 6.3 | 12.8 | 19.2 | 0.17 |
| ØCL 10 days after AI (mm) | 18.8 ± 0.6 | 18.7 ± 0.5 | 19.1 ± 0.5 | 0.71 |
| P/AI 30 days (%) | 64.0 | 70.0 | 61.0 | 0.70 |
| P/AI 45 days (%) | 62.0 | 65.0 | 61.0 | 0.93 |
| EM (%) | 3.0 | 6.0 | 0.0 | 0.30 |
- Cyclicity – buffalo considered cyclic when the presence of CL at the intravaginal device implant and/or removal moment. ØDF – diameter of dominant follicle. Growth rate of DF – calculated as the difference between the ØDF measured at TAI moment and the ØDF measured at P4 device removal moment. Ov – ovulation rate; Ovulation before AI – buffalo had dominant follicles ≥ 7 mm at Day 9 and a CL 10 days after TAI in the same ovary, however, no follicles ≥ 5 mm at the moment of AI (Day 12). ØCL – diameter of corpus luteum 10 days after TAI. P/IA 30 days – pregnancy per AI 30 days after TAI. P/IA 45 days – pregnancy per AI 45 days after TAI. EM – embryo or embryo heartbeat absence at 45 days after TAI, compared to the total females who had heart-beating embryos at 30 days.
As shown in Table 1, no differences in Ov (P = 0.69), P/AI 30 days (P = 0.70) and P/AI 45 days (P = 0.93), or EM (P = 0.30) among the treatments were observed. Despite similar responses of Ov and P/AI among treatments, there was a significant positive correlation between ØDF and these response variables. The ovulation rate showed a correlation of r = +0.44 (P < 0.01) with ØDF on Day 9, and r = +0.59 (P < 0.01) with ØDF on Day 12. Similarly, P/AI 45 days showed a correlation of r = +0.28 (P < 0.01) with ØDF on Day 9, and r = +0.49 (P < 0.01) with ØDF on Day 12. These correlations allowed the establishment of Ov occurrence and pregnancy probability curves between ØDF on Day 9 and Day 12 variables (Fig. 2).
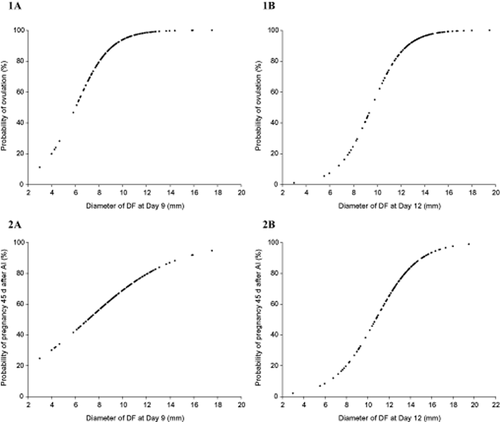
Probability of ovulation 10 days after artificial insemination (AI) (Panels 1A, 1B) and the probability of pregnancy 45 days after AI (Panels 2A, 2B) in grazing dairy buffalo cows (n = 142) submitted to E2 plus P4 based timed AI (TAI) protocols during the breeding season (autumn–winter), according to the diameter of the dominant follicle (ØDF) at P4 device removal (Panels 1A, 2A; Day 9) and at TAI (Panels 1B, 2B; Day 12). Panel 1A – Probability of ovulation = exp(−4.1536 + 0.6873*ØDFDay9)/1 + exp(−4.1536 + 0.6873*ØDF Day 9); P < 0.01. Panel 1B – Probability of ovulation = exp(−6.8746 + 0.7199*ØDF Day 12)/1 + exp(−6.8746 + 0.7199*ØDF Day 12); P < 0.01.Panel 2A – Probability of pregnancy = exp(−1.9404 + 0.2729*ØDF Day 9)/1 + exp(−1.9404 + 0.2729*ØDF Day 9); P < 0.01. Panel 2B – Probability of pregnancy = exp(−5.4272 + 0.5029*ØDF Day 12)/1 + exp(−5.4272 + 0.5029*ØDF Day 12); P < 0.01.
Based on the effect of ØDF on the probability of occurrence of ovulation and pregnancy in addition to the linear effect of CLD9 observed over ØDF on Day 9 (P = 0.09), the database was divided according to the presence or absence of a CL on Day 9 (CLD9 (91/142) or No-CLD9 (51/142)). As shown in Table 2, the animals that presented a CL at P4 device removal had lower ØDF on Day 9.
| Item | CL at P4 device remove (Day 9) | P-values | |
|---|---|---|---|
| CLD9 | No-CLD9 | ||
| No. of cows | 91 | 51 | |
| ØDF at Day 9 (mm) | 8.8 ± 0.2 | 9.9 ± 0.4 | 0.03 |
| ØDF at Day 12 (mm) | 12.2 ± 0.3 | 12.8 ± 0.4 | 0.40 |
| Growth rate of DF (mm/day) | 1.8 ± 0.1 | 1.8 ± 0.1 | 0.58 |
| Ovulation rate (%) | 80.2% | 86.3% | 0.64 |
| Ovulation before AI (%) | 11.0% | 15.7% | 0.46 |
| ØCL 10 days afterAI (mm) | 18.9 ± 0.4 | 18.9 ± 0.5 | 0.90 |
| P/AI 30 days (%) | 62.6% | 70.6% | 0.48 |
| P/AI 45 days (%) | 60.4% | 68.6% | 0.48 |
| EM (%) | 3.5% | 2.8% | 0.96 |
- ØDF – diameter of dominant follicle. Growth rate of DF – calculated as the difference between the ØDF measured at TAI moment and the ØDF measured at P4 device removal moment. Ov – ovulation rate; Ovulation before AI – buffalo had dominant follicles ≥ 7 mm at Day 9 and a CL 10 days after TAI in the same ovary, however, no follicles ≥ 5 mm at the moment of AI (Day 12). ØCL – diameter of corpus luteum 10 days after TAI. P/IA 30 days – pregnancy per AI 30 days after TAI. P/IA 45 days – pregnancy per AI 45 days after TAI. EM – embryo or embryo heartbeat absence at 45 days after TAI, compared to the total females who had heart-beating embryos at 30 days.
Discussion
The present study demonstrated that dairy buffalo subjected to an E2 plus P4 TAI-based protocol in the breeding season have similar ovarian responses, regardless of the treatment with new or previously used P4 devices. In addition, the new P4 device was able to maintain similar ovulatory and pregnancy rates, which rejected the present hypothesis. Additionally, it was found that ØDF on Days 9 and 12 of TAI are directly related to the ovulation and pregnancy outcomes. However, even the presence of a CL negatively affects the follicular development until the removal of the exogenous P4 source; the CL is not sufficient to compromise the P/AI of grazing dairy buffalo females submitted to the E2 plus P4 TAI based protocol.
Previous research on Bos taurus beef (Dadarwal et al. 2013), Bos indicus cattle (Carvalho et al. 2008; Barreiros et al. 2014) and dairy buffalo (Mendes et al. 2014) demonstrate the influence of P4 on follicular development during the synchronization protocols to TAI. These studies have shown the impairment of growth of the dominant follicle by the presence of a pre-synchronized and mature CL during the maintenance of a new P4 device from the TAI protocol. In the present study, despite the high cyclicity rate (∼80%), different P4 devices provided similar challenges for follicular development. Therefore, more important than the number of uses of a P4 device seems to be the presence of a CL at the end of the synchronization protocol, at the moment when the presence of a dominant follicle in the final stage of development is expected.
Similar ovarian follicular, ovulatory and pregnancy responses were found among grazing buffalo cows treated with different types of P4 devices. These results are comparable to those found by other researchers working with Bos indicus (Almeida et al. 2006; Meneghetti et al. 2009; Carneiro et al. 2012), crossbred beef heifers (Colazo et al. 2004; Mantovani et al. 2010) and Bos taurus dairy cows (Cerri et al. 2009). Similar results of follicular development, ovulatory and pregnancy responses were observed in anestrous buffalo treated with reused P4 devices during the off-breeding season (Carvalho et al. 2014). These results suggested that treatment of buffalo cows with a new or used P4 device during the breeding season provides sufficient control of follicular growth to prevent premature ovulation and ensure satisfactory ovarian responses following the E2 plus P4 TAI protocol.
When observing the probability curves, the presence of a larger DF on Days 9 and 12 increases the likelihood of ovulation and P/AI at 45 days. The association between ØDF and the risk of the occurrence of ovulation and/or pregnancy has been frequently reported in Bos taurus dairy cows (Vasconcelos et al. 2001; Pereira et al. 2013, 2014), Bos taurus beef heifers and cows (Perry et al. 2005, 2007), and Bos indicus beef heifers and cows (Sá Filho et al. 2010b,c, 2011). Although estradiol was not measured in this experiment, it is plausible that a higher concentration of this hormone in females is associated with larger follicles at the end of the synchronization protocols (Pandey et al. 2011). Furthermore, the estradiol milieu during proestrus has a positive effect on the establishment and maintenance of pregnancy in cattle (Santos et al. 2004; Jinks et al. 2012). Therefore, the development of the synchronization of ovulation protocols for TAI in buffalo dairy cows should focus on establishing larger and healthy follicles at the end of the synchronization treatment because doing so increases the likelihood of ovulation and pregnancy following the TAI.
The presence of a CL at P4 device removal demonstrated an influence on the ØDF on Day 9 but not on the ØDF on Day 12 (TAI). Similar findings of ØDF at P4 device removal were described by Sá Filho et al. (2010c) after TAI in Bos indicus heifers with or without a CL at the onset of the synchronization protocol. Similar to the present study, Sá Filho et al. (2010a,c) treated females with eCG at P4 device removal. In those studies, the smallest follicles were most likely stimulated to grow, achieving an adequate size to produce a satisfactory ovulatory response following the synchronization protocol. The beneficial effect of eCG has also been described in grazing dairy buffalo (Carvalho et al. 2013). That study found that eCG-treated females presented larger dominant follicle diameters and greater ovulatory and pregnancy responses compared with No-eCG-treated females.
Although LH pulses were not quantified in the current study, it is very likely that higher LH frequencies accounted for the higher follicular growth rate in buffalo of the No-CLD9 group. An alternative strategy for improving the final follicular growth is to reduce the P4 concentrations during the TAI protocols. Such a reduction could be achieved by the strategic administration of a PGF2α dose before the P4 device removal, as previously described in dairy cows (Pereira et al. 2013) and beef cattle (Carvalho et al. 2008; Peres et al. 2009; Dadarwal et al. 2013). Earlier luteolysis allows for higher LH frequency (Adams et al. 2008), leading to a large pre-ovulatory follicle (Carvalho et al. 2008) and, consequently, to a greater likelihood of pregnancy (Peres et al. 2009; Dadarwal et al. 2013; Pereira et al. 2013). This strategy certainly should be further evaluated in cyclic dairy buffalo cows subjected to E2 plus P4 TAI protocols during the breeding season.
In conclusion, regardless of the treatment with a new or a previously used P4 device, satisfactory ovarian responses were achieved when grazing dairy buffalo cows were subjected to an E2 plus P4 based TAI protocol during the breeding season. The presence of a larger follicle at P4 device removal and at TAI was associated with a higher ovulation rate and P/AI following TAI. Therefore, methods that increase the DF diameter by TAI may be important for improving the fertility of grazing dairy buffalo subjected to E2 plus P4 based TAI programs.
Acknowledgments
The authors are thankful to the commercial buffalo farms (Santa Helena, Guaviruva and Rincão) for allowing the use of the animals and their facilities. In addition, we are grateful to CAPES and OUROFINO Agribusiness (Cravinhos, Brazil) for the financial support.




