Effects of selenium supplementation on plasma progesterone concentrations in pregnant heifers
Abstract
It is known that selenium (Se) has various functions in animals. Many investigations on the biochemical and physiological effects of Se have been previously reported; however, the detailed function of Se in reproduction is not yet clear. We proposed the possibility that Se plays a notable role in progesterone production. The aim of this study was to clarify the effects of Se supplementation on progesterone levels of pregnant Holstein heifers. Eight Holstein heifers (−Se) were fed basal diet (containing 0.022 ppm of Se) throughout the experiment. While a 0.3 ppm diet of Se (sodium selenite) was fed to another seven animals (+Se) with basal diet. Blood sampling was carried out every week. Plasma Se concentrations were higher in Se-supplemented cows compared with controls (−Se) (P < 0.01) throughout the experiment. Se supplementation increased plasma progesterone in the 29–39 weeks of pregnancy from 4.98 ± 0.64 to 6.86 ± 0.49 ng/mL on average (P < 0.05). The present findings suggest that Se contributes to maintaining the function of the corpus luteum and/or placenta in the latter period of pregnancy.
Introduction
Since the essentiality of selenium (Se) in animals was verified by Schwarz and Foltz (1957), various nutritional functions of Se have been clarified. For example, Se contributes to the prevention of exudative diathesis (Bartholomew et al. 1998) and white muscle disease (Hooven et al. 2006), enhancement of cell-mediated and humoral immunity (Panousis et al. 2000; Spears 2000; Kamada et al. 2007), anticarcinogenesis (Kellen et al. 2006), alleviation of heavy metal toxicity (Bolkent et al. 2007; Falnoga & Tusek-Znidaric 2007) and improvement of reproductive performance (Harrison et al. 1984; Aréchiga et al. 1994, 1998; Kommisrud et al. 2005; Boitani & Puglisi 2008; Moeini et al. 2009). Harrison et al. reported that prepartum Se injections to dairy cows were effective in reducing the incidence of metritis and cystic ovaries during the early postpartum period. Aréchiga et al. showed the decrease of the incidence of retained fetal membranes and the increase of pregnancy rates by prepartum supplementation of vitamin E and Se to cows.
Several Se-containing enzymes have been identified as a result of biochemical studies: the first enzyme, glutathione peroxidase, showed the role of Se as an antioxidant; however, our understanding of the detailed metabolic role of Se in some organs is still limited despite vigorous research into the functions of seleno-enzymes. An experiment using 75Se in rats showed that Se is preferentially absorbed into endocrine and reproductive organs, including the corpus luteum (Behne et al. 1988), suggesting that Se could play an important role in these organs. Subsequently, a new seleno-enzyme, iodothyronine deiodinase, was discovered in the thyroid gland (Berry et al. 1991). Additionally, the lower serum insulin levels and abnormal pancreatic islet beta cells observed in Se-deficient animals suggested the importance of Se in pancreatic insulin secretion (Tong & Wang 1998).
It is known that Se supplementation increases the fertility of female animals; however, its mechanism is not yet clear. We have already proposed a hypothesis regarding the function of Se in the corpus luteum, based on the results of in vitro experiments (Kamada & Ikumo 1997). The corpus luteum is a tissue which produces a large amount of progesterone to maintain pregnancy. It is made from cholesterol by the reactions of many enzymes, including a side-chain cleavage enzyme which uses molecular oxygen for its reactions. It is known that these reactions easily produce oxygen radicals and various peroxides that are toxic to cells (Larroque et al. 1990). Our in vitro experiment showed that luteinizing hormone addition to luteal cell culture simultaneously increased progesterone concentration of the medium and the amount of lipid peroxides in cells (Kamada & Ikumo 1997). The accumulation of H2O2 (Riley & Behrman 1991) or lipid peroxides (Sawada & Carlson 1991) in the corpus luteum at functional luteal regression has been reported. These data indicate that the corpus luteum needs a defense system against such peroxides to maintain normal functions. The importance of antioxidants in corpus luteum has also been proposed by Carlson et al. (1995). Furthermore, we showed that Se addition to the luteal cell culture decreased the amount of lipid peroxides in a cell (Kamada & Ikumo 1997). Selenium as a component of glutathione peroxidase (antioxidant enzyme) may degrade peroxides, together with other scavenging systems, including superoxide dismutase, vitamin E and beta-carotene.
We have already reported that Se supplementation to non-pregnant cows increased their plasma progesterone concentration in the estrous cycle (Kamada & Hodate 1998). In this experiment, the effects of Se supplementation on the plasma progesterone concentration of pregnant heifers were investigated.
Materials and Methods
Animals
This study was conducted on 15 Holstein heifers (22.7 ± 0.4 months) kept at our institute. The animals were assigned to two experimental groups (n = 7–8 heifers/ group) on the basis of their body weights (−Se: 386.0 ± 9.6 kg, +Se: 386.7 ± 10.1 kg). Timothy hay, alfalfa hay cubes, corn starch and skim milk (basal diet) were fed to the animals twice daily (Table 1). The feed supply amount (energy, protein and minerals) met the requirement for pregnant heifers (NRC 2001). The basal diet contained 0.022 ± 0.005 ppm Se on average throughout the experiment (Se requirement for dairy cattle recommended by the National Research Council is 0.3 ppm). A 0.3 ppm diet of Se was fed to the Se addition treatment group (+Se: n = 7) using sodium selenite. Selenium was added to each of the two daily feedings. Control heifers (−Se: n = 8) were fed basal diet throughout experiment period. Shortages of magnesium, copper, manganese, zinc and cobalt in the basal diet were compensated for by supplementation of their inorganic salts (chloride) in both treatment groups. Fresh water was freely available. The animals were given adequate exercise in an outdoor paddock. After adaptation to each experimental diet, artificial insemination (AI) of the animals was carried out when blood plasma Se concentration in the +Se group was significantly higher than that of control heifers (all heifers used in this experiment received semen derived from one bull). The interval from the start of adaptation to conception was 64.5 ± 9.1 days on average. The estrous cycles of animals before AI were regular (17–23 days). The experimental treatment was continued until delivery. Blood samples were collected from the jugular vein using heparinized tubes once a week before the morning feed. After centrifugation (1000 × g, 30 min), blood plasma was stored at −50°C until analysis. All procedures were approved by the Animal Care and Use Committee of the NARO Institute of Livestock and Grassland Science. This experiment compared Se-deficient heifers (control) with Se-adequate heifers on plasma progesterone levels during pregnancy.
| Diet composition (dry matter) | g |
|---|---|
| Timothy hay | 8004 |
| Alfalfa hay cube | 1330 |
| Corn starch + skim milk (60:40) | 524 |
Measurement of Se
A fluorometric method using 2,3-diaminonaphtalene (Sigma-Aldrich, St. Louis, MO, USA) was used to measure Se concentrations in samples (Hoffman et al. 1968). One milliliter of blood plasma, 1.0 g of placenta tissue, or 0.5–1.0 g of feed was digested with 8.0 mL nitric acid and 4.0 mL perchloric acid. The cooled digesta was mixed with 1 mL of 2.4N hydrogen chloride, and boiled in hot water. After cooling, 5.0 mL of 0.02 mol/L ethylenediaminetetraacetic acid (EDTA) and 24 g/L hydroxylamine solution mixture was added. The pH of mixture was adjusted to 1.0–1.5 by a pH meter. After the addition of 5.0 mL of 0.1 % 2,3-diaminonaphtalene to the solution, it was incubated at 50°C for 25 min, and then extracted with 10 mL of n-hexane using a separation funnel after cooling. The fluorescence intensity of the organic phase was measured at an excitation wavelength of 378 nm and emission of 525 nm using a spectrophotofluorometer (AMINCO-Bowman™, Silver Spring, MD, USA). The calibration curve was made from the data of sodium selenite solution which was treated with the aforementioned same process.
Measurement of progesterone
Plasma concentrations of progesterone were determined by the dextran-charcoal method (Mathieu et al. 1977) using antisera to progesterone (anti-P-3-OMO-BSA; Teikokuzoki Pharmaceuticals Co., Tokyo, Japan) and 3H-labeled progesterone (1,2,6,7-3H(N); Amersham Biosciences Corp., Piscataway, NJ, USA). A 10 μL plasma sample was mixed with 200 μL of diluted antibody. The next day, 100 μL of labeled progesterone was added to the mixture. On the final day, 500 μL of dextran-charcoal was used for bound/free separation. After centrifugation (1000 × g, 15 min) of the assay tube, radioactivity in the supernatant was measured by a liquid scintillation counter. Samples of the two treatments on the same day of pregnancy were allocated continuously and measured. Data are means in duplicate assays. The intra- and inter-assay coefficients of variation were 7.6% and 9.1%, respectively.
Measurement of alpha-tocopherol
Plasma concentration of alpha-tocopherol at 0 week, 20 week of pregnancy and delivery was analyzed using high pressure liquid chromatography (Japan Spectroscopic Co., Ltd, Tokyo, Japan). A 0.2 mL sample of plasma was mixed with 1.0 mL water and 1.0 mL ethanol, and extracted with 5.0 mL n-hexane. The elution profile was monitored at an excitation wavelength of 298 nm and emission of 325 nm.
Statistical analysis
Progesterone data were categorized into two periods (0–28 weeks and 29–39 weeks) with or without the hormonal contribution of placenta on pregnancy. Blood Se data and area under the curve (AUC) values of progesterone were subjected to analysis of variance (ANOVA) using the GLM procedure (SAS Institute 2008). Significance was considered at P < 0.05 and P < 0.01.
Results
Plasma concentration of Se
After animal adaptation to the experimental diets, plasma concentration of Se was greater (P < 0.01) in +Se (80.6 ± 3.8) compared with –Se (31.6 ± 3.0) heifers (Fig. 1). This difference continued up to calving (P < 0.001). Se concentrations in Se-supplemented animals (+Se) were kept at a sufficient concentration (> 70 ppb), whereas those in control animals (−Se) decreased gradually, reaching the concentration of Se deficiency (< 40 ppb) throughout pregnancy (Puls 1981).
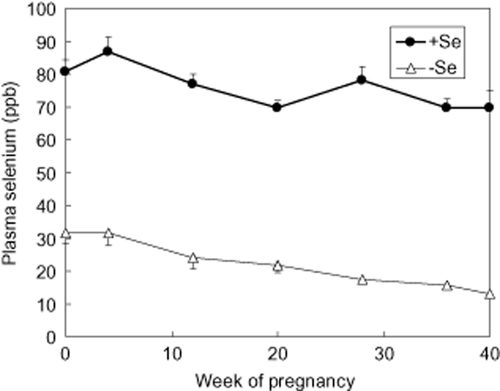
Effect of selenium (Se) supplementation to the diet on plasma selenium concentration. A 0.3 ppm diet of Se (sodium selenite) was fed to Se-addition treatment cows (n = 7) until delivery. Artificial insemination (AI) of the animals (n = 15) was carried out after adaptation to the experimental diet. Blood samples were collected from the jugular vein and centrifuged. The selenium concentration of plasma was measured by a fluorometric method using 2,3-diaminonaphtalene. Data represent the means with standard error for seven or eight animals in each treatment. Significance was considered at P < 0.01.
Plasma progesterone concentration
The average lengths of gestation were 278.3 ± 1.5 (+Se) and 269.8 ± 8.0 (−Se), respectively. (One heifer in the control group aborted at 215 days of pregnancy. Pathologic findings of white muscle disease were not observed in the aborted calf.) Figure 2 shows the changes in plasma progesterone concentration during gestation. It is known that a hormonal contribution of corpus luteum (including placenta) on pregnancy is different before and after 29 weeks of pregnancy (Tanabe 1966; Estergreen Jr et al. 1967; Chew et al. 1979), so data was analyzed on two periods (0–28 weeks, 29–39 weeks), respectively. Although average AUC values of plasma progesterone concentration was not different between the two treatments during 0-28 week of pregnancy, Se supplementation significantly increased in the last 10 weeks (29–39 weeks of pregnancy) (P < 0.05). Average concentrations of progesterone during this period were 4.98 ± 0.64 ng/mL in the control group (−Se) and 6.86 ± 0.49 ng/mL in the treated group (+Se), respectively.
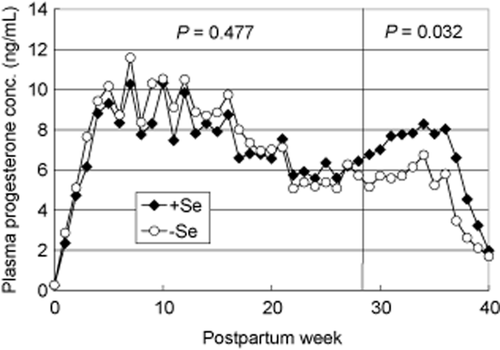
Effect of selenium supplementation to the diet on plasma progesterone concentration. The progesterone concentration of blood plasma was measured by radio-immunoassay using the dextran-charcoal method. Blood samples were collected once a week. Data represent the means with standard error for seven or eight animals in each treatment every week. Significance was considered at P < 0.05.
Plasma alpha-tocopherol concentration
The average concentrations of plasma alpha-tocopherol at 0 week, 20 weeks and delivery in the two groups were 1.20 ± 0.17, 1.47 ± 0.08, 1.33 ± 0.11 μg/mL (−Se) and 1.07 ± 0.11, 1.44 ± 0.22, 1.02 ± 0.10 μg/mL (+Se), respectively, which were not different between the two treatments. These values represent a sufficient concentration (above 1.0 μg/mL) for dairy cattle (NRC 1987).
Discussion
In this experiment, we showed that differences in dietary Se concentration affected plasma progesterone concentrations in the latter period of bovine pregnancy. We have already demonstrated that luteal cells easily produce lipid peroxides together with progesterone production (Kamada & Ikumo 1997). So we inferred that, in Se-deficient cows, the accumulation of peroxides in the corpus luteum damaged its function and decreased the concentration of plasma progesterone. The accumulation of a large amount of peroxides in the corpus luteum leads to luteal regression and abortion (Kodaman et al. 1994). Thus Se supplementation to pregnant cows could contribute to maintaining pregnancy by decreasing the risk of luteal regression, and decreasing the risk of premature birth. However, in this study alpha-tocopherol levels in the plasma of heifers were sufficient regardless of treatment, so it was likely that a shortage of Se in control animals did not cause serious problems. Double deficiency (Se and alpha-tocopherol) may cause significant disorders of progesterone production, while the effect of Se observed in alpha-tocopherol sufficiency indicated that target molecules on the antioxidant system are different between Se and alpha-tocopherol. The measurement of peroxides in the corpus luteum would have been most interesting; however, biopsy was not carried out because it would inevitably damage the luteal function of the experimental animals. Investigation of Se effect on a healthy corpus luteum was most important in this research.
Although we have reported the positive effects of Se on plasma progesterone concentration in the estrous cycle and the progesterone production of luteal cells (Kamada & Ikumo 1997; Kamada & Hodate 1998), the importance of extra-luteal sources of progesterone in pregnancy has also been reported. It is known that some cows ovariectomized after 200 days (28 weeks) of pregnancy can maintain their pregnancy (Conley & Ford 1987; Shemesh 1990; Willard et al. 2005). In these cases, progesterone source instead of corpus luteum is considered to be placenta and/or adrenals; however, progesterone supply from these organs is likely to be a subsidiary system when the corpus luteum function is injured. Most cows ovariectomized after 200 days of pregnancy could not perform normal gestation (shortened gestation) (Stabenfeldt et al. 1970), and had high risks of dystocia, retained placenta, metritis and high mortality of fetuses and calves. Moreover, the suppression of progesterone production of placenta by the corpus luteum and small amounts of progesterone production in the placenta in normal pregnancy were reported (Johnson et al. 1981; Conley & Ford 1987). These details suggest the leading role of progesterone derived from the corpus luteum even in the latter period of pregnancy. It is expected that long-term progesterone production in the corpus luteum throughout pregnancy leads to the accumulation of large amounts of peroxide and that the detoxifying effect of Se resolves disordered corpus luteum function by peroxides during late pregnancy. Our data do not reject the possibility of Se function in the placenta or adrenals, and our hypothesis (degradation of peroxides by Se) can be applied to these organs; however, a large amount of peroxides will not be accumulated in these organs because active progesterone production in the placenta or adrenals is shorter in time than that in the corpus luteum. Thus the corpus luteum is considered to be a powerful target tissue of Se effect on progesterone production. Nevertheless Se concentration of discharged placenta in Se-supplemented heifers was significantly higher than that in control heifers (−Se: 50.5 ± 5.9 ppb, + Se: 94.3 ± 7.8 ppb, P < 0.01), so the function of Se in placenta on progesterone production cannot be denied. (Preferential absorption of Se into the corpus luteum and adrenals was also reported by Behne et al. (1988).
Another possibility of Se function on plasma progesterone concentration is a decrease of progesterone catabolism; however, we do not have any data about this point. Steroid hormones are dissimilated in liver and excreted via bile; however, Sakuma et al. (2007) have reported that Se status does not affect the composition of bile. Further, if Se suppressed progesterone clearance, plasma progesterone concentration would increase throughout the whole experimental period; however, such an effect was not observed in this experiment. These details suggest that Se does not affect progesterone clearance in the pregnancy period.
Selenium may play an important role in the organs relating to corpus luteum function, such as the pituitary gland or hypothalamus, because it is known that the pituitary gland and brain also preferentially retain Se (Behne et al. 1988); however, we do not have any additional data relevant to this possibility at the present time. In the kidney, which was shown to absorb Se preferentially in the same report (Behne et al. 1988), a new seleno-enzyme, thyroxin deiodinase, was discovered. So it is possible that an unidentified seleno-protein may act in the pituitary gland or hypothalamus.
It has been reported that plasma progesterone concentrations in cows with a retained placenta (RP) in late pregnancy were relatively lower than those of normal cows without RP (Estergreen Jr et al. 1967; Ishikawa et al. 2004), stated that the fetal membranes were retained in nearly all animals after removal of the corpus luteum in the second half of pregnancy. We showed that the dietary Se level affects progesterone concentration in this period. The RP-decreasing effect of Se may be the result of an increase in progesterone concentration in response to this element. However, in this experiment RP was not observed even in −Se treatment heifers. It is known that alpha-tocopherol partly compensates for Se function. Supplementation with Se and alpha-tocopherol is more effective in decreasing the incidence of RP (Eger et al. 1985). Given that plasma alpha-tocopherol was at sufficient levels in this experiment, its presence might have prevented this reproductive disorder.
No effect of dietary Se supplementation on progesterone concentration in blood plasma was observed in the early period of pregnancy. Although we have reported that Se supplementation to non-pregnant cows immediately increased the plasma progesterone concentration in the estrous cycle (Kamada & Hodate 1998), the present result (not immediate reaction) in pregnant heifers appears to be inconsistent. However, the qualitative difference in the antioxidant system between the corpus luteum in the estrous cycle and pregnancy might affect the results of progesterone concentration in early pregnancy. It is known that some antioxidant systems actively work in the corpus luteum during pregnancy. Sugino et al. (2000) reported that Cu, Zn-SOD activities in the corpus luteum of pregnant humans were significantly higher than during the menstrual cycle, and significant increases in glutathione peroxidase activities in microsomes and the mitochondrial fraction from the corpus luteum of pregnant pigs were recorded by other researchers (Eliasson et al. 1999). Moreover, it has been reported that the highest beta-carotene (antioxidant) levels in the plasma, corpus luteum, and follicular fluid were found during pregnancy when there is maximal luteal function (Haliloglu et al. 2002). The corpus luteum during pregnancy could have various defense systems to protect against oxidative damage and maintain its function for a long time (about 280 days); however, as gestation progresses, peroxides saved from detoxification by antioxidant systems might accumulate in the corpus luteum and cause functional disorder, resulting in lower progesterone concentration in the latter period of pregnancy as shown in control animals. We inferred that Se suppressed the accumulation of a large amount of peroxide in the corpus luteum mainly in the latter period of pregnancy, as a component of glutathione peroxidase. Ovariectomy after 200 days of pregnancy shortened the average gestation length, and caused uterine inertia, partial cervical dilation and fetal membrane retention (Johnson et al. 1981), and many calves born to ovariectomized cows were weak and died after delivery (Stabenfeldt et al. 1970). These data indicate that an adequate level of progesterone in the latter half of pregnancy is needed to complete a normal gestation. Selenium could contribute to maintain an adequate level of progesterone in this period.
Acknowledgments
The authors wish to thank the staff of the Ruminant and Field Management Section of the NARO Institute of Livestock and Grassland Sciences for their assistance with animal handling and care. We would also like to thank Dr. Hitomi Takahashi, Dr. Manabu Shimizu and Dr. Minoru Narita for their technical advice. And we express our gratitude to Dr. Fuminori Terada and Dr. Osamu Sasaki for their statistical analysis.




